A bacterial group II intron encoding reverse transcriptase, maturase, and DNA endonuclease activities: biochemical demonstration of maturase activity and insertion of new genetic information within the intron
- PMID: 9353259
- PMCID: PMC316661
- DOI: 10.1101/gad.11.21.2910
A bacterial group II intron encoding reverse transcriptase, maturase, and DNA endonuclease activities: biochemical demonstration of maturase activity and insertion of new genetic information within the intron
Abstract
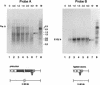
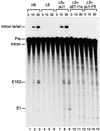
The Lactococcus lactis group II intron Ll.ltrB is similar to mobile yeast mtDNA group II introns, which encode reverse transcriptase, RNA maturase, and DNA endonuclease activities for site-specific DNA insertion. Here, we show that the Lactococcal intron can be expressed and spliced efficiently in Escherichia coli. The intron-encoded protein LtrA has reverse transcriptase and RNA maturase activities, with the latter activity shown both in vivo and in vitro, a first for any group II intron-encoded protein. As for the yeast mtDNA introns, the DNA endonuclease activity of the Lactococcal intron is associated with RNP particles containing both the intron-encoded protein and the excised intron RNA. Also, the intron RNA cleaves the sense-strand of the recipient DNA by a reverse splicing reaction, whereas the intron-encoded protein cleaves the antisense strand. The Lactococcal intron endonuclease can be obtained in large quantities by coexpression of the LtrA protein with the intron RNA in E. coli or reconstituted in vitro by incubating the expressed LtrA protein with in vitro-synthesized intron RNA. Furthermore, the specificity of the endonuclease and reverse splicing reactions can be changed predictably by modifying the RNA component. Expression in E. coli facilitates the use of group II introns for the targeting of specific foreign sequences to a desired site in DNA.
Figures








References
-
- Belfort M, Ehrenman K, Chandry PS. Genetic and molecular analysis of RNA splicing in Escherichia coli. Methods Enzymol. 1990;181:521–539. - PubMed
-
- Bonen L. Trans-splicing of pre-mRNA in plants, animals, and protists. FASEB J. 1993;7:40–46. - PubMed
-
- Caprara MG, Mohr G, Lambowitz AM. A tyrosyl-tRNA synthetase protein induces tertiary folding of the group I intron catalytic core. J Mol Biol. 1996;257:512–531. - PubMed
-
- Carignani G, Groudinsky O, Frezza D, Schiavon E, Bergantino E, Slonimski P. An mRNA maturase is encoded by the first intron of the mitochondrial gene for the subunit I of cytochrome oxidase in S. cerevisiae. Cell. 1983;35:733–742. - PubMed
-
- Collins ML, Hunsaker WR. Improved hybridization assays employing tailed oligonucleotide probes: A direct comparison with 5′-end-labeled oligonucleotide probes and nick-translated plasmid probes. Anal Biochem. 1985;151:211–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
