The ribosome-in-pieces: binding of elongation factor EF-G to oligoribonucleotides that mimic the sarcin/ricin and thiostrepton domains of 23S ribosomal RNA
- PMID: 9356440
- PMCID: PMC24907
- DOI: 10.1073/pnas.94.23.12280
The ribosome-in-pieces: binding of elongation factor EF-G to oligoribonucleotides that mimic the sarcin/ricin and thiostrepton domains of 23S ribosomal RNA
Abstract
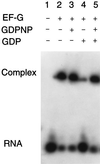
An oligoribonucleotide (a 27-mer) that mimics the sarcin/ricin (S/R) domain of Escherichia coli 23S rRNA binds elongation factor EF-G; the Kd is 6.9 microM, whereas for binding to ribosomes it is 0.7 microM. Binding saturates when EF-G and the S/R RNA are equimolar; at saturation 70% of the input RNA is in complexes with EF-G. Binding of EF-G to S/R RNA does not require GTP but is inhibited by GDP; the inhibition by GDP is overcome by GTP. The effects of mutations of the S/R domain nucleotides G2655, A2660, and G2661 suggest that EF-G recognizes the conformation of the RNA rather than the identity of the nucleotides. EF-G also binds to an oligoribonucleotide (an 84-mer) that has the thiostrepton region of 23S rRNA; however, EF-G binds independently to S/R and thiostrepton oligoribonucleotides.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

