Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution
- PMID: 9356455
- PMCID: PMC24947
- DOI: 10.1073/pnas.94.23.12366
Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution
Abstract
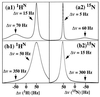
Fast transverse relaxation of 1H, 15N, and 13C by dipole-dipole coupling (DD) and chemical shift anisotropy (CSA) modulated by rotational molecular motions has a dominant impact on the size limit for biomacromolecular structures that can be studied by NMR spectroscopy in solution. Transverse relaxation-optimized spectroscopy (TROSY) is an approach for suppression of transverse relaxation in multidimensional NMR experiments, which is based on constructive use of interference between DD coupling and CSA. For example, a TROSY-type two-dimensional 1H,15N-correlation experiment with a uniformly 15N-labeled protein in a DNA complex of molecular mass 17 kDa at a 1H frequency of 750 MHz showed that 15N relaxation during 15N chemical shift evolution and 1HN relaxation during signal acquisition both are significantly reduced by mutual compensation of the DD and CSA interactions. The reduction of the linewidths when compared with a conventional two-dimensional 1H,15N-correlation experiment was 60% and 40%, respectively, and the residual linewidths were 5 Hz for 15N and 15 Hz for 1HN at 4 degrees C. Because the ratio of the DD and CSA relaxation rates is nearly independent of the molecular size, a similar percentagewise reduction of the overall transverse relaxation rates is expected for larger proteins. For a 15N-labeled protein of 150 kDa at 750 MHz and 20 degrees C one predicts residual linewidths of 10 Hz for 15N and 45 Hz for 1HN, and for the corresponding uniformly 15N,2H-labeled protein the residual linewidths are predicted to be smaller than 5 Hz and 15 Hz, respectively. The TROSY principle should benefit a variety of multidimensional solution NMR experiments, especially with future use of yet somewhat higher polarizing magnetic fields than are presently available, and thus largely eliminate one of the key factors that limit work with larger molecules.
Figures



References
-
- Shulman R G, Ogawa S, Wüthrich K, Yamane T, Peisach J, Blumberg W E. Science. 1969;165:251–257. - PubMed
-
- Arata Y, Kato K, Takahashi H, Shimada I. Methods Enzymol. 1994;239:440–464. - PubMed
-
- Wüthrich K. NMR of Proteins and Nucleic Acids. New York: Wiley; 1986.
-
- Wüthrich K. NMR in Structural Biology. Singapore: World Scientific; 1995.
-
- Wüthrich K. Acta Cryst D. 1995;51:249–270. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

