Nonmuscle myosin II-B is required for normal development of the mouse heart
- PMID: 9356462
- PMCID: PMC24969
- DOI: 10.1073/pnas.94.23.12407
Nonmuscle myosin II-B is required for normal development of the mouse heart
Abstract
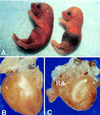
We used targeted gene disruption in mice to ablate nonmuscle myosin heavy chain B (NMHC-B), one of the two isoforms of nonmuscle myosin II present in all vertebrate cells. Approximately 65% of the NMHC-B-/- embryos died prior to birth, and those that were born suffered from congestive heart failure and died during the first day. No abnormalities were detected in NMHC-B+/- mice. The absence of NMHC-B resulted in a significant increase in the transverse diameters of the cardiac myocytes from 7.8 +/- 1.8 micron (right ventricle) and 7.8 +/- 1.3 micron (left ventricle) in NMHC-B+/+ and B+/- mice to 14.7 +/- 1.1 micron and 13.8 +/- 2.3 micron, respectively, in NMHC-B-/- mice (in both cases, P < 0.001). The increase in size of the cardiac myocytes was seen as early as embryonic day 12.5 (4.5 +/- 0.2 micron for NMHC-B+/+ and B+/- vs. 7. 2 +/- 0.6 micron for NMHC-B-/- mice (P < 0.01)). Six of seven NMHC-B-/- newborn mice analyzed by serial sectioning also showed structural cardiac defects, including a ventricular septal defect, an aortic root that either straddled the defect or originated from the right ventricle, and muscular obstruction to right ventricular outflow. Some of the hearts of NMHC-B-/- mice showed evidence for up-regulation of NMHC-A protein. These studies suggest that nonmuscle myosin II-B is required for normal cardiac myocyte development and that its absence results in structural defects resembling, in part, two common human congenital heart diseases, tetralogy of Fallot and double outlet right ventricle.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

