Dystrobrevin and dystrophin: an interaction through coiled-coil motifs
- PMID: 9356463
- PMCID: PMC24974
- DOI: 10.1073/pnas.94.23.12413
Dystrobrevin and dystrophin: an interaction through coiled-coil motifs
Abstract
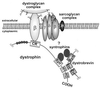
Dystrobrevin, a dystrophin-related and -associated protein, has been proposed to be important in the formation and maintenance of the neuromuscular junction. Dystrobrevin coprecipitates with both the acetylcholine receptor complex as well as the dystrophin glycoprotein complex. Although the nature of dystrobrevin's association with the dystrophin glycoprotein complex remains unclear, it is known that dystrobrevin binds directly to the syntrophins, a heterologous group of dystrophin-associated proteins. Using the yeast two-hybrid system to identify protein-protein interactions, we present evidence for the heterodimerization of dystrobrevin directly with dystrophin. The C terminus of dystrobrevin binds specifically to the C terminus of dystrophin. We further refined this site of interaction to these proteins' homologous coiled-coil motifs that flank their respective syntrophin-binding sites. We also show that the interaction between the dystrobrevin and dystrophin coiled-coil domains is specific and is not due to a nonspecific coiled-coil domain interaction. From the accumulated evidence of protein-protein interactions presented here and elsewhere, we propose a partially revised model of the organization of the dystrophin-associated glycoprotein complex.
Figures


References
-
- Wagner K R, Cohen J B, Huganir R L. Neuron. 1993;10:511–522. - PubMed
-
- Butler M H, Douville K, Murnane A A, Kramarcy N R, Cohen J B, Sealock R, Froehner S C. J Biol Chem. 1992;267:6213–6218. - PubMed
-
- Ervasti J M, Campbell K P. Cell. 1991;66:1121–1131. - PubMed
-
- Ibraghimov-Beskrovnaya O, Ervasti J M, Leveille C J, Slaughter C A, Sernett S W, Campbell K P. Nature (London) 1992;355:696–702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

