Parasite-mediated nuclear factor kappaB regulation in lymphoproliferation caused by Theileria parva infection
- PMID: 9356483
- PMCID: PMC25026
- DOI: 10.1073/pnas.94.23.12527
Parasite-mediated nuclear factor kappaB regulation in lymphoproliferation caused by Theileria parva infection
Abstract
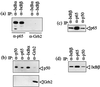
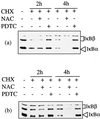
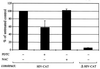
Infection of cattle with the protozoan Theileria parva results in uncontrolled T lymphocyte proliferation resulting in lesions resembling multicentric lymphoma. Parasitized cells exhibit autocrine growth characterized by persistent translocation of the transcriptional regulatory factor nuclear factor kappaB (NFkappaB) to the nucleus and consequent enhanced expression of interleukin 2 and the interleukin 2 receptor. How T. parva induces persistent NFkappaB activation, required for T cell activation and proliferation, is unknown. We hypothesized that the parasite induces degradation of the IkappaB molecules which normally sequester NFkappaB in the cytoplasm and that continuous degradation requires viable parasites. Using T. parva-infected T cells, we showed that the parasite mediates continuous phosphorylation and proteolysis of IkappaBalpha. However, IkappaBalpha reaccumulated to high levels in parasitized cells, which indicated that T. parva did not alter the normal NFkappaB-mediated positive feedback loop which restores cytoplasmic IkappaBalpha. In contrast, T. parva mediated continuous degradation of IkappaBbeta resulting in persistently low cytoplasmic IkappaBbeta levels. Normal IkappaBbeta levels were only restored following T. parva killing, indicating that viable parasites are required for IkappaBbeta degradation. Treatment of T. parva-infected cells with pyrrolidine dithiocarbamate, a metal chelator, blocked both IkappaB degradation and consequent enhanced expression of NFkappaB dependent genes. However treatment using the antioxidant N-acetylcysteine had no effect on either IkappaB levels or NFkappaB activation, indicating that the parasite subverts the normal IkappaB regulatory pathway downstream of the requirement for reactive oxygen intermediates. Identification of the critical points regulated by T. parva may provide new approaches for disease control as well as increase our understanding of normal T cell function.
Figures



Similar articles
-
The intracellular parasite Theileria parva protects infected T cells from apoptosis.Proc Natl Acad Sci U S A. 1999 Jun 22;96(13):7312-7. doi: 10.1073/pnas.96.13.7312. Proc Natl Acad Sci U S A. 1999. PMID: 10377411 Free PMC article.
-
Differential in vitro and in vivo expression of MHC class II antigens in bovine lymphocytes infected by Theileria parva.Vet Immunol Immunopathol. 1993 Jan;35(3-4):253-73. doi: 10.1016/0165-2427(93)90038-6. Vet Immunol Immunopathol. 1993. PMID: 8430496
-
Interference by the intracellular parasite Theileria parva with T-cell signal transduction pathways induces transformation and protection against apoptosis.Vet Immunol Immunopathol. 1999 Dec 15;72(1-2):95-100. doi: 10.1016/s0165-2427(99)00121-x. Vet Immunol Immunopathol. 1999. PMID: 10614498 Review.
-
Infection with the intracellular protozoan parasite Theileria parva induces constitutively high levels of NF-kappa B in bovine T lymphocytes.Mol Cell Biol. 1989 Nov;9(11):4677-86. doi: 10.1128/mcb.9.11.4677-4686.1989. Mol Cell Biol. 1989. PMID: 2513476 Free PMC article.
-
Theileria parva: taking control of host cell proliferation and survival mechanisms.Cell Microbiol. 2000 Apr;2(2):91-9. doi: 10.1046/j.1462-5822.2000.00045.x. Cell Microbiol. 2000. PMID: 11207566 Review.
Cited by
-
NF-kappaB family of transcription factors: central regulators of innate and adaptive immune functions.Clin Microbiol Rev. 2002 Jul;15(3):414-29. doi: 10.1128/CMR.15.3.414-429.2002. Clin Microbiol Rev. 2002. PMID: 12097249 Free PMC article. Review.
-
Transcriptomics reveal potential vaccine antigens and a drastic increase of upregulated genes during Theileria parva development from arthropod to bovine infective stages.PLoS One. 2018 Oct 10;13(10):e0204047. doi: 10.1371/journal.pone.0204047. eCollection 2018. PLoS One. 2018. PMID: 30303978 Free PMC article.
-
East Coast Fever Caused by Theileria parva Is Characterized by Macrophage Activation Associated with Vasculitis and Respiratory Failure.PLoS One. 2016 May 19;11(5):e0156004. doi: 10.1371/journal.pone.0156004. eCollection 2016. PLoS One. 2016. PMID: 27195791 Free PMC article.
-
Transient efficacy of buparvaquone against the US isolate of Theileria orientalis Ikeda genotype in sub-clinically infected cattle.Front Vet Sci. 2024 Jul 26;11:1421710. doi: 10.3389/fvets.2024.1421710. eCollection 2024. Front Vet Sci. 2024. PMID: 39132441 Free PMC article.
-
Modulation of tumor necrosis factor by microbial pathogens.PLoS Pathog. 2006 Feb;2(2):e4. doi: 10.1371/journal.ppat.0020004. PLoS Pathog. 2006. PMID: 16518473 Free PMC article. Review.
References
-
- Ruben S, Poteat H, Tan T H, Kawakami K, Roeder R, Haseltine W, Rosen C A. Science. 1988;241:89–92. - PubMed
-
- Ballard D W, Bohnlein E, Lowenthal J W, Wano Y, Franza B R, Greene W C. Science. 1988;241:1652–1655. - PubMed
-
- Crenon I, Beraud C, Simard P, Montagne J, Veschambre P, Jalinot P. Oncogene. 1993;8:867–875. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

