Effects of in vivo adventitial expression of recombinant endothelial nitric oxide synthase gene in cerebral arteries
- PMID: 9356490
- PMCID: PMC25041
- DOI: 10.1073/pnas.94.23.12568
Effects of in vivo adventitial expression of recombinant endothelial nitric oxide synthase gene in cerebral arteries
Abstract
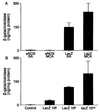
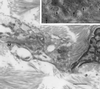
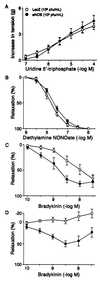
Nitric oxide (NO), synthesized from L-arginine by NO synthases (NOS), plays an essential role in the regulation of cerebrovascular tone. Adenoviral vectors have been widely used to transfer recombinant genes to different vascular beds. To determine whether the recombinant endothelial NOS (eNOS) gene can be delivered in vivo to the adventitia of cerebral arteries and functionally expressed, a replication-incompetent adenoviral vector encoding eNOS gene (AdCMVNOS) or beta-galactosidase reporter gene (AdCMVLacZ) was injected into canine cerebrospinal fluid (CSF) via the cisterna magna (final viral titer in CSF, 10(9) pfu/ml). Adventitial transgene expression was demonstrated 24 h later by beta-galactosidase histochemistry and quantification, eNOS immunohistochemistry, and Western blot analysis of recombinant eNOS. Electron microscopy immunogold labeling indicated that recombinant eNOS protein was expressed in adventitial fibroblasts. In AdCMVNOS-transduced arteries, basal cGMP production and bradykinin-induced relaxations were significantly augmented when compared with AdCMVLacZ-transduced vessels (P < 0.05). The increased receptor-mediated relaxations and cGMP production were inhibited by eNOS inhibitors. In addition, the increase in cGMP production was reversed in the absence of calcium, suggesting that the increased NO production did not result from inducible NOS expression. The present study demonstrates the successful in vivo transfer and functional expression of recombinant eNOS gene in large cerebral arteries. It also suggests that perivascular eNOS gene delivery via the CSF is a feasible approach that does not require interruption of cerebral blood flow.
Figures



Similar articles
-
Adventitial expression of recombinant eNOS gene restores NO production in arteries without endothelium.Arterioscler Thromb Vasc Biol. 1998 Aug;18(8):1231-41. doi: 10.1161/01.atv.18.8.1231. Arterioscler Thromb Vasc Biol. 1998. PMID: 9714129
-
Adenovirus-mediated gene transfer to human cerebral arteries.J Cereb Blood Flow Metab. 2000 Sep;20(9):1360-71. doi: 10.1097/00004647-200009000-00011. J Cereb Blood Flow Metab. 2000. PMID: 10994858
-
Expression and function of recombinant endothelial nitric oxide synthase gene in canine basilar artery.Circ Res. 1997 Mar;80(3):327-35. doi: 10.1161/01.res.80.3.327. Circ Res. 1997. PMID: 9048652
-
Adenovirus-mediated gene transfer to cerebral circulation.Mech Ageing Dev. 2000 Jul 31;116(2-3):95-101. doi: 10.1016/s0047-6374(00)00123-8. Mech Ageing Dev. 2000. PMID: 10996009 Review.
-
Dysfunction of nitric oxide synthases as a cause and therapeutic target in delayed cerebral vasospasm after SAH.Neurol Res. 2006 Oct;28(7):730-7. doi: 10.1179/016164106X152052. Neurol Res. 2006. PMID: 17164036 Review.
Cited by
-
Communication Is Key: Mechanisms of Intercellular Signaling in Vasodilation.J Cardiovasc Pharmacol. 2017 May;69(5):264-272. doi: 10.1097/FJC.0000000000000463. J Cardiovasc Pharmacol. 2017. PMID: 28482351 Free PMC article. Review.
-
Hypertension, stroke, and endothelium.Curr Hypertens Rep. 2005 Feb;7(1):68-71. doi: 10.1007/s11906-005-0057-5. Curr Hypertens Rep. 2005. PMID: 15683589 Review.
-
Gene therapy for cerebral vascular disease: update 2003.Br J Pharmacol. 2003 May;139(1):1-9. doi: 10.1038/sj.bjp.0705217. Br J Pharmacol. 2003. PMID: 12746217 Free PMC article. Review.
-
Gene transfer for cerebrovascular disease.Curr Cardiol Rep. 2001 Jan;3(1):10-6. doi: 10.1007/s11886-001-0004-2. Curr Cardiol Rep. 2001. PMID: 11139793 Review.
-
Transcriptional targeting of tumor endothelial cells for gene therapy.Adv Drug Deliv Rev. 2009 Jul 2;61(7-8):542-53. doi: 10.1016/j.addr.2009.02.006. Epub 2009 Apr 23. Adv Drug Deliv Rev. 2009. PMID: 19393703 Free PMC article. Review.
References
-
- Edvinsson L, MacKenzie E T, McCulloch J, editors. Cerebral Blood Flow and Metabolism. New York, NY: Raven; 1993.
-
- Heistad D D, Faraci F M. Stroke. 1996;27:1688–1693. - PubMed
-
- Ooboshi H, Welsh M J, Rios C D, Davidson B L, Heistad D D. Circ Res. 1995;77:7–13. - PubMed
-
- Muhonen M G, Ooboshi H, Welsh M J, Davidson B L, Heistad D D. Stroke. 1997;28:822–829. - PubMed
-
- Furchgott R F. J Am Med Assoc. 1996;276:1186–1188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

