The affected gene underlying the class K glycosylphosphatidylinositol (GPI) surface protein defect codes for the GPI transamidase
- PMID: 9356492
- PMCID: PMC25045
- DOI: 10.1073/pnas.94.23.12580
The affected gene underlying the class K glycosylphosphatidylinositol (GPI) surface protein defect codes for the GPI transamidase
Abstract
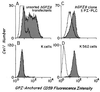
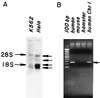
The final step in glycosylphosphatidylinositol (GPI) anchoring of cell surface proteins consists of a transamidation reaction in which preassembled GPI donors are substituted for C-terminal signal sequences in nascent polypeptides. In previous studies we described a human K562 cell mutant, termed class K, that accumulates fully assembled GPI units but is unable to transfer them to N-terminally processed proproteins. In further work we showed that, unlike wild-type microsomes, microsomes from these cells are unable to support C-terminal interaction of proproteins with the small nucleophiles hydrazine or hydroxylamine, and that the cells thus are defective in transamidation. In this study, using a modified recombinant vaccinia transient transfection system in conjunction with a composite cDNA prepared by 5' extension of an existing GenBank sequence, we found that the genetic element affected in these cells corresponds to the human homolog of yGPI8, a gene affected in a yeast mutant strain exhibiting similar accumulation of GPI donors without transfer. hGPI8 gives rise to mRNAs of 1.6 and 1.9 kb, both encoding a protein of 395 amino acids that varies in cells with their ability to couple GPIs to proteins. The gene spans approximately 25 kb of DNA on chromosome 1. Reconstitution of class K cells with hGPI8 abolishes their accumulation of GPI precursors and restores C-terminal processing of GPI-anchored proteins. Also, hGPI8 restores the ability of microsomes from the mutant cells to yield an active carbonyl in the presence of a proprotein which is considered to be an intermediate in catalysis by a transamidase.
Figures




References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

