Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein
- PMID: 9356502
- PMCID: PMC25065
- DOI: 10.1073/pnas.94.23.12638
Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein
Abstract
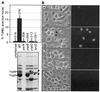
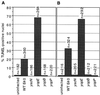
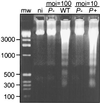
Yersiniae, causative agents of plague and gastrointestinal diseases, secrete and translocate Yop effector proteins into the cytosol of macrophages, leading to disruption of host defense mechanisms. It is shown in this report that Yersinia enterocolitica induces apoptosis in macrophages and that this effect depends on YopP. Functional secretion and translocation mechanisms are required for YopP to act, strongly suggesting that this protein exerts its effect intracellularly, after translocation into the macrophages. YopP shows a high level of sequence similarity with AvrRxv, an avirulence protein from Xanthomonas campestris, a plant pathogen that induces programmed cell death in plant cells. This indicates possible similarities between the strategies used by pathogenic bacteria to elicit programmed cell death in both plant and animal hosts.
Figures
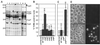

Similar articles
-
YopJ-induced caspase-1 activation in Yersinia-infected macrophages: independent of apoptosis, linked to necrosis, dispensable for innate host defense.PLoS One. 2012;7(4):e36019. doi: 10.1371/journal.pone.0036019. Epub 2012 Apr 26. PLoS One. 2012. PMID: 22563435 Free PMC article.
-
Evidence for targeting of Yop effectors by the chromosomally encoded Ysa type III secretion system of Yersinia enterocolitica.J Bacteriol. 2002 Oct;184(20):5563-71. doi: 10.1128/JB.184.20.5563-5571.2002. J Bacteriol. 2002. PMID: 12270813 Free PMC article.
-
Reduced secretion of YopJ by Yersinia limits in vivo cell death but enhances bacterial virulence.PLoS Pathog. 2008 May 16;4(5):e1000067. doi: 10.1371/journal.ppat.1000067. PLoS Pathog. 2008. PMID: 18483548 Free PMC article.
-
Crosstalk of signalling processes of innate immunity with Yersinia Yop effector functions.Immunobiology. 2008;213(3-4):261-9. doi: 10.1016/j.imbio.2007.11.001. Epub 2007 Dec 31. Immunobiology. 2008. PMID: 18406372 Review.
-
Role of macrophage apoptosis in the pathogenesis of Yersinia.Curr Top Microbiol Immunol. 2005;289:151-73. doi: 10.1007/3-540-27320-4_7. Curr Top Microbiol Immunol. 2005. PMID: 15791955 Review.
Cited by
-
Epithelial cells infected with Chlamydophila pneumoniae (Chlamydia pneumoniae) are resistant to apoptosis.Infect Immun. 2001 Dec;69(12):7880-8. doi: 10.1128/IAI.69.12.7880-7888.2001. Infect Immun. 2001. PMID: 11705971 Free PMC article.
-
Structure-function analysis of the Shigella virulence factor IpaB.J Bacteriol. 2001 Feb;183(4):1269-76. doi: 10.1128/JB.183.4.1269-1276.2001. J Bacteriol. 2001. PMID: 11157939 Free PMC article.
-
Dating the origin of the CCR5-Delta32 AIDS-resistance allele by the coalescence of haplotypes.Am J Hum Genet. 1998 Jun;62(6):1507-15. doi: 10.1086/301867. Am J Hum Genet. 1998. PMID: 9585595 Free PMC article.
-
SseL, a Salmonella deubiquitinase required for macrophage killing and virulence.Proc Natl Acad Sci U S A. 2007 Feb 27;104(9):3502-7. doi: 10.1073/pnas.0610095104. Epub 2007 Feb 20. Proc Natl Acad Sci U S A. 2007. PMID: 17360673 Free PMC article.
-
YopR impacts type III needle polymerization in Yersinia species.Mol Microbiol. 2010 Jan;75(1):221-9. doi: 10.1111/j.1365-2958.2009.06988.x. Epub 2009 Dec 7. Mol Microbiol. 2010. PMID: 19968786 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

