Galectin-4 and small intestinal brush border enzymes form clusters
- PMID: 9362066
- PMCID: PMC25705
- DOI: 10.1091/mbc.8.11.2241
Galectin-4 and small intestinal brush border enzymes form clusters
Abstract
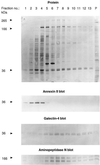
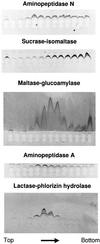
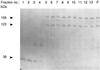
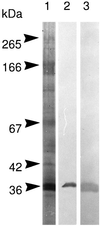
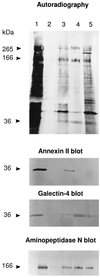
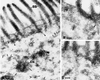
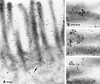
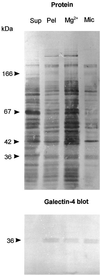
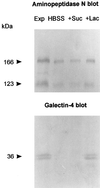
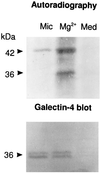
Detergent-insoluble complexes prepared from pig small intestine are highly enriched in several transmembrane brush border enzymes including aminopeptidase N and sucrase-isomaltase, indicating that they reside in a glycolipid-rich environment in vivo. In the present work galectin-4, an animal lectin lacking a N-terminal signal peptide for membrane translocation, was discovered in these complexes as well, and in gradient centrifugation brush border enzymes and galectin-4 formed distinct soluble high molecular weight clusters. Immunoperoxidase cytochemistry and immunogold electron microscopy showed that galectin-4 is indeed an intestinal brush border protein; we also localized galectin-4 throughout the cell, mainly associated with membraneous structures, including small vesicles, and to the rootlets of microvillar actin filaments. This was confirmed by subcellular fractionation, showing about half the amount of galectin-4 to be in the microvillar fraction, the rest being associated with insoluble intracellular structures. A direct association between the lectin and aminopeptidase N was evidenced by a colocalization along microvilli in double immunogold labeling and by the ability of an antibody to galectin-4 to coimmunoprecipitate aminopeptidase N and sucrase-isomaltase. Furthermore, galectin-4 was released from microvillar, right-side-out vesicles as well as from mucosal explants by a brief wash with 100 mM lactose, confirming its extracellular localization. Galectin-4 is therefore secreted by a nonclassical pathway, and the brush border enzymes represent a novel class of natural ligands for a member of the galectin family. Newly synthesized galectin-4 is rapidly "trapped" by association with intracellular structures prior to its apical secretion, but once externalized, association with brush border enzymes prevents it from being released from the enterocyte into the intestinal lumen.
Figures


References
-
- Alpers DH. In: Physiology of the Gastrointestinal Tract. L.R. Johnson, New York: Raven Press; 1987. pp. 1469–1487.
-
- Barondes SH, Cooper DNW, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269:20807–20810. - PubMed
-
- Bjerrum OJ, Larsen KP, Wilkien M. In: Modern Methods in Protein Chemistry. Tschesche H, editor. Berlin: Walter de Gruyter; 1983. pp. 79–124.
-
- Booth AG, Kenny AJ. A morphometric and biochemical investigation of the vesiculation of kidney microvilli. J Cell Sci. 1976;21:449–463. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

