Three-dimensional residual strain in midanterior canine left ventricle
- PMID: 9362268
- PMCID: PMC3343007
- DOI: 10.1152/ajpheart.1997.273.4.H1968
Three-dimensional residual strain in midanterior canine left ventricle
Abstract
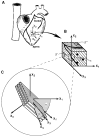
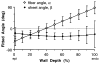
All previous studies of residual strain in the ventricular wall have been based on one- or two-dimensional measurements. Transmural distributions of three-dimensional (3-D) residual strains were measured by biplane radiography of columns of lead beads implanted in the midanterior free wall of the canine left ventricle (LV). 3-D bead coordinates were reconstructed with the isolated arrested LV in the zero-pressure state and again after local residual stress had been relieved by excising a transmural block of tissue. Nonhomogeneous 3-D residual strains were computed by finite element analysis. Mean +/- SD (n = 8) circumferential residual strain indicated that the intact unloaded myocardium was prestretched at the epicardium (0.07 +/- 0.06) and compressed in the subendocardium (-0.04 +/- 0.05). Small but significant longitudinal shortening and torsional shear residual strains were also measured. Residual fiber strain was tensile at the epicardium (0.05 +/- 0.06) and compressive in the subendocardium (-0.01 +/- 0.04), with residual extension and shortening, respectively, along structural axes parallel and perpendicular to the laminar myocardial sheets. Relatively small residual shear strains with respect to the myofiber sheets suggest that prestretching in the plane of the myocardial laminae may be a primary mechanism of residual stress in the LV.
Figures




References
-
- De Tombe PP, ter Keurs HEDJ. Sarcomere dynamics in cat cardiac trabeculae. Circ Res. 1991;68:588–596. - PubMed
-
- Fung YC. Biomechanics: Motion, Flow, Stress, Growth. New York: Springer-Verlag; 1990.
-
- Grimm AF, Katele KV, Lin HL. Fiber bundle direction in the mammalian heart. Basic Res Cardiol. 1976;71:381–388. - PubMed
-
- Guccione JM, McCulloch AD, Waldman LK. Passive material properties of intact ventricular myocardium determined from a cylindrical model. J Biomech Eng. 1991;113:42–55. - PubMed
-
- Hunter PJ, Nielsen PMF, Smaill BH, LeGrice IJ, Hunter IW. An anatomical heart model with applications to myocardial activation and ventricular mechanics. Crit Rev Biomed Eng. 1992;20:403–426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

