Differential localization of vesicular acetylcholine and monoamine transporters in PC12 cells but not CHO cells
- PMID: 9362509
- PMCID: PMC2139959
- DOI: 10.1083/jcb.139.4.907
Differential localization of vesicular acetylcholine and monoamine transporters in PC12 cells but not CHO cells
Abstract
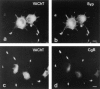
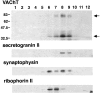
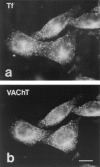
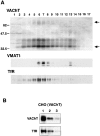
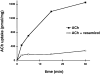
Previous studies have indicated that neuro-endocrine cells store monoamines and acetylcholine (ACh) in different secretory vesicles, suggesting that the transport proteins responsible for packaging these neurotransmitters sort to distinct vesicular compartments. Molecular cloning has recently demonstrated that the vesicular transporters for monoamines and ACh show strong sequence similarity, and studies of the vesicular monoamine transporters (VMATs) indicate preferential localization to large dense core vesicles (LDCVs) rather than synaptic-like microvesicles (SLMVs) in rat pheochromocytoma PC12 cells. We now report the localization of the closely related vesicular ACh transporter (VAChT). In PC12 cells, VAChT differs from the VMATs by immunofluorescence and fractionates almost exclusively to SLMVs and endosomes by equilibrium sedimentation. Immunoisolation further demonstrates colocalization with synaptophysin on SLMVs as well as other compartments. However, small amounts of VAChT also occur on LDCVs. Thus, VAChT differs in localization from the VMATs, which sort predominantly to LDCVs. In addition, we demonstrate ACh transport activity in stable PC12 transformants overexpressing VAChT. Since previous work has suggested that VAChT expression confers little if any transport activity in non-neural cells, we also determined its localization in transfected CHO fibroblasts. In CHO cells, VAChT localizes to the same endosomal compartment as the VMATs by immunofluorescence, density gradient fractionation, and immunoisolation with an antibody to the transferrin receptor. We have also detected ACh transport activity in the transfected CHO cells, indicating that localization to SLMVs is not required for function. In summary, VAChT differs in localization from the VMATs in PC12 cells but not CHO cells.
Figures
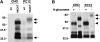







Similar articles
-
A phosphorylation site regulates sorting of the vesicular acetylcholine transporter to dense core vesicles.J Cell Biol. 2000 Apr 17;149(2):379-96. doi: 10.1083/jcb.149.2.379. J Cell Biol. 2000. PMID: 10769030 Free PMC article.
-
Visualization of the vesicular acetylcholine transporter in cholinergic nerve terminals and its targeting to a specific population of small synaptic vesicles.Proc Natl Acad Sci U S A. 1996 Apr 16;93(8):3547-52. doi: 10.1073/pnas.93.8.3547. Proc Natl Acad Sci U S A. 1996. PMID: 8622973 Free PMC article.
-
Preferential localization of a vesicular monoamine transporter to dense core vesicles in PC12 cells.J Cell Biol. 1994 Dec;127(5):1419-33. doi: 10.1083/jcb.127.5.1419. J Cell Biol. 1994. PMID: 7962100 Free PMC article.
-
Transport mechanisms in acetylcholine and monoamine storage.FASEB J. 2000 Dec;14(15):2423-34. doi: 10.1096/fj.00-0203rev. FASEB J. 2000. PMID: 11099460 Review.
-
Molecular analysis of vesicular amine transporter function and targeting to secretory organelles.FASEB J. 2000 Dec;14(15):2450-8. doi: 10.1096/fj.00-0206rev. FASEB J. 2000. PMID: 11099462 Review.
Cited by
-
A tyrosine-based motif localizes a Drosophila vesicular transporter to synaptic vesicles in vivo.J Biol Chem. 2010 Mar 5;285(10):6867-78. doi: 10.1074/jbc.M109.073064. Epub 2010 Jan 6. J Biol Chem. 2010. PMID: 20053989 Free PMC article.
-
A phosphorylation site regulates sorting of the vesicular acetylcholine transporter to dense core vesicles.J Cell Biol. 2000 Apr 17;149(2):379-96. doi: 10.1083/jcb.149.2.379. J Cell Biol. 2000. PMID: 10769030 Free PMC article.
-
Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans.Nat Med. 2011 Jun 19;17(7):888-92. doi: 10.1038/nm.2371. Nat Med. 2011. PMID: 21685896 Free PMC article.
-
Synaptic vesicle transporter expression regulates vesicle phenotype and quantal size.J Neurosci. 2000 Oct 1;20(19):7297-306. doi: 10.1523/JNEUROSCI.20-19-07297.2000. J Neurosci. 2000. PMID: 11007887 Free PMC article.
-
Signals involved in targeting membrane proteins to synaptic vesicles.Cell Mol Neurobiol. 2002 Dec;22(5-6):565-77. doi: 10.1023/a:1021884319363. Cell Mol Neurobiol. 2002. PMID: 12585680 Free PMC article. Review.
References
-
- Alfonso A, Grundahl K, Duerr JS, Han H-P, Rand JB. The Caenorhabditis elegans unc-17 gene: a putative vesicular acetylcholine transporter. Science. 1993;261:617–619. - PubMed
-
- Anderson DC, King SC, Parsons SM. Proton gradient linkage to active uptake of 3H-acetylcholine by Torpedo electric organ synaptic vesicles. Biochemistry. 1982;21:3037–3043. - PubMed
-
- Bauerfeind R, Regnier-Vigouroux A, Flatmark T, Huttner WB. Selective storage of acetylcholine, but not catecholamines, in neuroendocrine synaptic-like microvesicles of early endosomal origin. Neuron. 1993;11:105–121. - PubMed
-
- Bejanin S, Cervini R, Mallet J, Berrard S. A unique gene organization for two cholinergic markers, choline acetyltransferase and a putative vesicular transporter of acetylcholine. J Biol Chem. 1994;269:21944–21947. - PubMed
-
- Blumberg D, Schweitzer ES. Vesamicol binding to subcellular membranes that are distinct from catecholaminergic vesicles in PC12 cells. J Neurochem. 1992;58:801–810. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

