XMAP310: a Xenopus rescue-promoting factor localized to the mitotic spindle
- PMID: 9362515
- PMCID: PMC2139961
- DOI: 10.1083/jcb.139.4.975
XMAP310: a Xenopus rescue-promoting factor localized to the mitotic spindle
Abstract
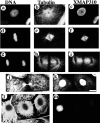
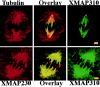
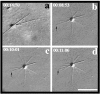
To understand the role of microtubule-associated proteins (MAPs) in the regulation of microtubule (MT) dynamics we have characterized MAPs prepared from Xenopus laevis eggs (Andersen, S.S.L., B. Buendia, J.E. Domínguez, A. Sawyer, and E. Karsenti. 1994. J. Cell Biol. 127:1289-1299). Here we report on the purification and characterization of a 310-kD MAP (XMAP310) that localizes to the nucleus in interphase and to mitotic spindle MTs in mitosis. XMAP310 is present in eggs, oocytes, a Xenopus tissue culture cell line, testis, and brain. We have purified XMAP310 to homogeneity from egg extracts. The purified protein cross-links pure MTs. Analysis of the effect of this protein on MT dynamics by time-lapse video microscopy has shown that it increases the rescue frequency 5-10-fold and decreases the shrinkage rate twofold. It has no effect on the growth rate or the catastrophe frequency. Microsequencing data suggest that XMAP230 and XMAP310 are novel MAPs. Although the three Xenopus MAPs characterized so far, XMAP215 (Vasquez, R.J., D.L. Gard, and L. Cassimeris. 1994. J. Cell Biol. 127:985-993), XMAP230, and XMAP310 are localized to the mitotic spindle, they have distinct effects on MT dynamics. While XMAP215 promotes rapid MT growth, XMAP230 decreases the catastrophe frequency and XMAP310 increases the rescue frequency. This may have important implications for the regulation of MT dynamics during spindle morphogenesis and chromosome segregation.
Figures



References
-
- Andersen SSL, Ashford AJ, Tournebize R, Gavet O, Sobel A, Hyman AA, Karsenti E. Mitotic chromatin regulates phosphorylation of Stathmin/Op18. Nature. 1997;389:640–643. - PubMed
-
- Ashford, A.J., S.S.L. Andersen, and A.A. Hyman. 1998. Purification of bovine brain tubulin. In Cell Biology: A Laboratory Handbook. J.E. Celis, editor. Academic Press, San Diego. Vol. II. 205–212.
-
- Belmont LD, Mitchison TJ. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell. 1996;84:623–631. - PubMed
-
- Belmont LD, Hyman AA, Sawin KE, Mitchison TJ. Real-time visualization of cell cycle-dependent changes in microtubule dynamics in cytoplasmic extracts. Cell. 1990;62:579–589. - PubMed

