Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells
- PMID: 9362528
- PMCID: PMC2199138
- DOI: 10.1084/jem.186.10.1677
Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells
Abstract
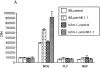
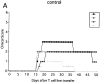
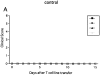
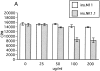
In this report, we establish a regulatory role of natural killer (NK) cells in experimental autoimmune encephalomyelitis (EAE), a prototype T helper cell type 1 (Th1)-mediated disease. Active sensitization of C57BL/6 (B6) mice with the myelin oligodendrocyte glycoprotein (MOG)35-55 peptide induces a mild form of monophasic EAE. When mice were deprived of NK cells by antibody treatment before immunization, they developed a more serious form of EAE associated with relapse. Aggravation of EAE by NK cell deletion was also seen in beta 2-microglobulin-/- (beta 2m-/-) mice, indicating that NK cells can play a regulatory role in a manner independent of CD8+ T cells or NK1.1+ T cells (NK-T cells). The disease enhancement was associated with augmentation of T cell proliferation and production of Th1 cytokines in response to MOG35-55. EAE passively induced by the MOG35-55-specific T cell line was also enhanced by NK cell deletion in B6, beta 2m-/-, and recombination activation gene 2 (RAG-2)-/- mice, indicating that the regulation by NK cells can be independent of T, B, or NK-T cells. We further showed that NK cells inhibit T cell proliferation triggered by antigen or cytokine stimulation. Taken together, we conclude that NK cells are an important regulator for EAE in both induction and effector phases.
Figures



















References
-
- Experimental allergic encephalomyelitis: a useful model for multiple sclerosis. 1984. E.C. Alvord, Jr., M.W. Kies, and A.J. Suckling, editors. Alan R. Liss, New York.
-
- Tabira, T., and J. Kira. 1992. Strain and species differences of encephalitogenic determinants of myelin basic protein and proteolipid apoprotein. In Myelin: Biology and Chemistry. R.E. Martenson, editor. CRC Press Inc., Boca Raton, FL. 783–799.
-
- Chen Y, Kuchroo VK, Inobe J-I, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science (Wash DC) 1994;265:1237–1240. - PubMed
-
- Sun D, Ben-Nun A, Wekerle H. Regulatory circuits in autoimmunity: recruitment of counter-regulatory CD8+ T cells by encephalitogenic CD4+T line cells. Eur J Immunol. 1988;18:1993–1999. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

