Regulation of ZAP-70 intracellular localization: visualization with the green fluorescent protein
- PMID: 9362531
- PMCID: PMC2199132
- DOI: 10.1084/jem.186.10.1713
Regulation of ZAP-70 intracellular localization: visualization with the green fluorescent protein
Abstract
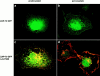
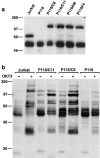
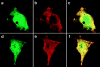
To investigate the cellular dynamics of ZAP-70, we have studied the distribution and regulation of its intracellular location using a ZAP-70 green fluorescent protein chimera. Initial experiments in epithelial cells indicated that ZAP-70 is diffusely located throughout the quiescent cell, and accumulates at the plasma membrane upon cellular activation, a phenotype enhanced by the coexpression of Lck and the initiation of ZAP-70 kinase activity. Subsequent studies in T cells confirmed this phenotype. Intriguingly, a large amount of ZAP-70, both chimeric and endogenous, resides in the nucleus of quiescent and activated cells. Nuclear ZAP-70 becomes tyrosine phosphorylated upon stimulation via the T cell receptor, indicating that it may have an important biologic function.
Figures








References
-
- Samelson LE, Klausner RD. Tyrosine kinases and tyrosine-based activation motifs. J Biol Chem. 1992;267:24913–24916. - PubMed
-
- Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. - PubMed
-
- Iwashima M, Irving BA, van Oers NSC, Chan AC, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science (Wash DC) 1994;263:1136–1139. - PubMed
-
- Pawson T. Protein modules and signalling networks. Nature (Lond) 1995;373:573–580. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

