Knock-out mice reveal a critical antiepileptic role for neuropeptide Y
- PMID: 9364040
- PMCID: PMC6573608
- DOI: 10.1523/JNEUROSCI.17-23-08927.1997
Knock-out mice reveal a critical antiepileptic role for neuropeptide Y
Abstract
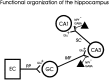
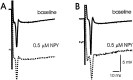
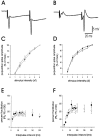
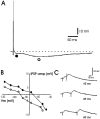
Neuropeptide Y (NPY) inhibits excitatory synaptic transmission in the hippocampus and is implicated in control of limbic seizures. In the present study, we examined hippocampal function and the response to pharmacologically induced seizures in mutant mice lacking this peptide. In slice electrophysiology studies, no change in normal hippocampal function was observed in NPY-deficient mice compared with normal wild-type littermates. Kainic acid (KA) produced limbic seizures at a comparable latency and concentration in NPY-deficient mice compared with littermates. However, KA-induced seizures progressed uncontrollably and ultimately produced death in 93% of NPY-deficient mice, whereas death was rarely observed in wild-type littermates. Intracerebroventricular NPY infusion, before KA administration, prevented death in NPY-deficient mice. These results suggest a critical role for endogenous NPY in seizure control.
Figures



References
-
- Allen YS, Adrian TE, Allen JM, Tatemoto K, Crow TJ, Bloom SR, Polak JM. Neuropeptide Y distribution in the rat brain. Science. 1983;221:877–879. - PubMed
-
- Andersen P, Bliss TVP, Lømo T, Olsen LI, Skrede KK. Lamellar organization of hippocampal excitatory pathways. Acta Physiol Scand. 1969;76:4A–5A. - PubMed
-
- Austin KB, Bronzino JD, Morgane PJ. Paired-pulse facilitation and inhibition in the dentate gyrus is dependent on behavioral state. Exp Brain Res. 1989;77:594–604. - PubMed
-
- Baraban SC, Schwartzkroin PA. Effects of prenatal cocaine exposure on the developing hippocampus: intrinsic and synaptic physiology. J Neurophysiol. 1997;77:126–136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
