Epsilon subunit-containing acetylcholine receptors in myotubes belong to the slowly degrading population
- PMID: 9364041
- PMCID: PMC6573614
- DOI: 10.1523/JNEUROSCI.17-23-08937.1997
Epsilon subunit-containing acetylcholine receptors in myotubes belong to the slowly degrading population
Abstract
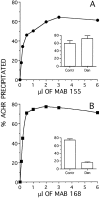
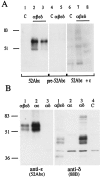
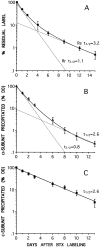
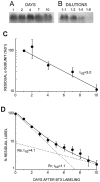
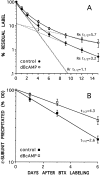
Two types of muscle acetylcholine receptors (AChRs) can be distinguished on the basis of their degradation rates and sensitivities to innervation, muscle activity, and agents elevating intracellular cAMP. The first type (Rs), is present in a stable form (degradation t1/2 = approximately 10 d) at the adult innervated neuromuscular junctions (NMJs). Rs can also exist in a less stable form (called accelerated Rs; t1/2 = approximately 3-5 d) at denervated NMJs and in aneurally cultured myotubes; agents that increase intracellular cAMP reversibly modulate Rs stability. The second type of AChR is a rapidly degrading receptor (Rr) expressed only in embryonic and noninnervated muscles. Rr can be stabilized by ATP and not by cAMP. This study tested the hypothesis that the degradation properties unique to the Rs are attributable to the presence of the epsilon subunit. Immunoprecipitation and Western blot analysis of AChRs extracted from rat muscle cells in tissue culture showed that AChRs recognized by antibodies against the epsilon subunit degraded as a single population with a half-life similar to that of the slow component, Rs, in these cells. In addition, as for Rs receptors in denervated NMJs and cultured muscle cell, the degradation rate of these epsilon-containing AChRs was stabilized by dibutyryl-cAMP. The data indicate that the epsilon-containing AChRs behave like Rs. Thus, the presence of the epsilon subunit is sufficient for selecting an AChR molecule to the Rs pool.
Figures
References
-
- Blount P, Merlie JP. Characterization of an adult muscle acetylcholine receptor subunit by expression in fibroblasts. J Biol Chem. 1991;266:14692–14696. - PubMed
-
- Brenner HR, Witzemann V, Sakmann B. Imprinting of acetylcholine receptor messenger RNA accumulation in mammalian neuromuscular synapses. Nature. 1990;344:544–547. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
