Development of intrinsic and synaptic properties in a forebrain nucleus essential to avian song learning
- PMID: 9364047
- PMCID: PMC6573603
- DOI: 10.1523/JNEUROSCI.17-23-08997.1997
Development of intrinsic and synaptic properties in a forebrain nucleus essential to avian song learning
Abstract
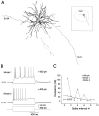
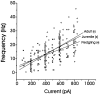
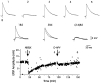
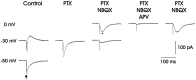
In male zebra finches, the lateral magnocellular nucleus of the anterior neostriatum (LMAN) is necessary for the development of learned song but is not required for the production of acoustically stereotyped (crystallized) adult song. One hypothesis is that the physiological properties of LMAN neurons change over development and thus limit the ability of LMAN to affect song. To test this idea, we used in vitro intracellular recordings to characterize the intrinsic and synaptic properties of LMAN neurons in fledgling [posthatch days (PHD) 22-32] and juvenile zebra finches (PHD 40-51) when LMAN lesions disrupt normal song development, and in adults (>PHD 90) when LMAN lesions are without effect. In fledglings, depolarizing currents caused LMAN projection neurons to fire bursts of action potentials because of a putative low-threshold calcium spike (LTS). In contrast, juvenile and adult LMAN projection neurons fired accommodating trains of action potentials when depolarized but did not exhibit the burst mode of firing. Electrical stimulation of thalamic afferents elicited both monosynaptic EPSPs mediated by AMPA and NMDA receptors and polysynaptic IPSPs mediated by GABAA receptors from LMAN neurons at all ages studied here. In whole-cell voltage-clamp recordings, the EPSCs (NMDA-EPSCs) consisted of fast and slow components. Unlike juvenile and adult NMDA-EPSCs, those in fledglings were dominated by the slower component. Thus, both the intrinsic and synaptic properties of LMAN neurons change markedly during early song development (PHD 22-40) and achieve several adult-like properties during early sensorimotor learning and well before the time when LMAN lesions no longer disrupt song development.
Figures






Similar articles
-
Silent synapses in a thalamo-cortical circuit necessary for song learning in zebra finches.J Neurophysiol. 2005 Dec;94(6):3698-707. doi: 10.1152/jn.00282.2005. Epub 2005 Aug 17. J Neurophysiol. 2005. PMID: 16107531
-
Androgens modulate NMDA receptor-mediated EPSCs in the zebra finch song system.J Neurophysiol. 1999 Nov;82(5):2221-34. doi: 10.1152/jn.1999.82.5.2221. J Neurophysiol. 1999. PMID: 10561401
-
Intrinsic and thalamic excitatory inputs onto songbird LMAN neurons differ in their pharmacological and temporal properties.J Neurophysiol. 1998 May;79(5):2615-28. doi: 10.1152/jn.1998.79.5.2615. J Neurophysiol. 1998. PMID: 9582233
-
Intrinsic and synaptic properties of neurons in an avian thalamic nucleus during song learning.J Neurophysiol. 2002 Oct;88(4):1903-14. doi: 10.1152/jn.2002.88.4.1903. J Neurophysiol. 2002. PMID: 12364516
-
Lateral magnocellular nucleus of the anterior neostriatum (LMAN) in the zebra finch: neuronal connectivity and the emergence of sex differences in cell morphology.Microsc Res Tech. 2001 Sep 15;54(6):335-53. doi: 10.1002/jemt.1147. Microsc Res Tech. 2001. PMID: 11668647 Review.
Cited by
-
Lesions of an avian forebrain nucleus that disrupt song development alter synaptic connectivity and transmission in the vocal premotor pathway.J Neurosci. 1999 Nov 1;19(21):9385-98. doi: 10.1523/JNEUROSCI.19-21-09385.1999. J Neurosci. 1999. PMID: 10531443 Free PMC article.
-
Activity propagation in an avian basal ganglia-thalamocortical circuit essential for vocal learning.J Neurosci. 2009 Apr 15;29(15):4782-93. doi: 10.1523/JNEUROSCI.4903-08.2009. J Neurosci. 2009. PMID: 19369547 Free PMC article.
-
Social context rapidly modulates the influence of auditory feedback on avian vocal motor control.J Neurophysiol. 2009 Oct;102(4):2485-97. doi: 10.1152/jn.00340.2009. Epub 2009 Aug 19. J Neurophysiol. 2009. PMID: 19692513 Free PMC article.
-
Development of individual axon arbors in a thalamocortical circuit necessary for song learning in zebra finches.J Neurosci. 2002 Feb 1;22(3):901-11. doi: 10.1523/JNEUROSCI.22-03-00901.2002. J Neurosci. 2002. PMID: 11826119 Free PMC article.
-
Exploring the molecular basis of neuronal excitability in a vocal learner.BMC Genomics. 2019 Aug 2;20(1):629. doi: 10.1186/s12864-019-5871-2. BMC Genomics. 2019. PMID: 31375088 Free PMC article.
References
-
- Aamodt SM, Kozlowski MR, Nordeen EJ, Nordeen KW. Distribution and developmental change in [3H]MK-801 binding within zebra finch song nuclei. J Neurobiol. 1992;23:997–1005. - PubMed
-
- Arnold AP. The effects of castration on song development in zebra finches (Poephila guttata). J Exp Zool. 1975;191:261–278. - PubMed
-
- Ball GF. Neurochemical specializations associated with vocal learning and production in songbirds and budgerigars. Brain Behav Evol. 1994;44:234–246. - PubMed
-
- Basham ME. Developmental regulation of mRNA for the NMDAR1 subunit in zebra finches. Soc Neurosci Abstr. 1996;22:692.
-
- Basham ME, Nordeen EJ, Nordeen KW. Blockade of NMDA receptors in the anterior forebrain impairs sensory acquisition in the zebra finch (Poephila guttata). Neurobiol Learn Mem. 1996;66:295–304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
