Mechanism and kinetics of heterosynaptic depression at a cerebellar synapse
- PMID: 9364051
- PMCID: PMC6573588
- DOI: 10.1523/JNEUROSCI.17-23-09048.1997
Mechanism and kinetics of heterosynaptic depression at a cerebellar synapse
Abstract
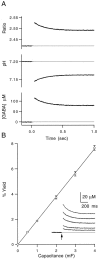
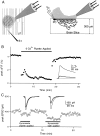
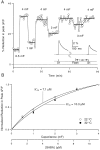
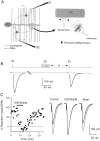
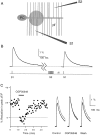
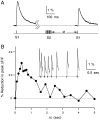
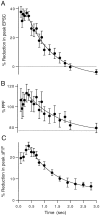
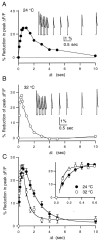
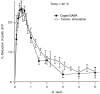
High levels of activity at a synapse can lead to spillover of neurotransmitter from the synaptic cleft. This extrasynaptic neurotransmitter can diffuse to neighboring synapses and modulate transmission via presynaptic receptors. We studied such modulation at the synapse between granule cells and Purkinje cells in rat cerebellar slices. Brief tetanic stimulation of granule cell parallel fibers activated inhibitory neurons, leading to a transient elevation of extracellular GABA, which in turn caused a short-lived heterosynaptic depression of the parallel fiber to Purkinje cell EPSC. Fluorometric calcium measurements revealed that this synaptic inhibition was associated with a decrease in presynaptic calcium influx. Heterosynaptic inhibition of synaptic currents and calcium influx was eliminated by antagonists of the GABAB receptor. The magnitude and time course of the depression of calcium influx were mimicked by the rapid release of an estimated 10 microM GABA using the technique of flash photolysis. We found that inhibition of presynaptic calcium influx peaked within 300 msec and decayed in <3 sec at 32 degrees C. These results indicate that presynaptic GABAB receptors can sense extrasynaptic GABA increases of several micromolar and that they rapidly regulate the release of neurotransmitter primarily by modulating voltage-gated calcium channels.
Figures



References
-
- Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61.
-
- Anwyl R. Modulation of vertebrate neuronal calcium channels by transmitters. Brain Res Rev. 1991;16:265–281. - PubMed
-
- Bonanno G, Raiteri M. Functional evidence for multiple GABAB receptor subtypes in the rat cerebral cortex. J Pharmacol Exp Ther. 1992;262:114–118. - PubMed
-
- Bonanno G, Raiteri M. Multiple GABAB receptors. Trends Pharmacol Sci. 1993;14:259–261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
