Pyruvate protects neurons against hydrogen peroxide-induced toxicity
- PMID: 9364052
- PMCID: PMC6573585
- DOI: 10.1523/JNEUROSCI.17-23-09060.1997
Pyruvate protects neurons against hydrogen peroxide-induced toxicity
Abstract
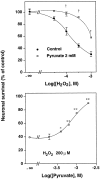
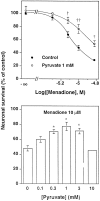
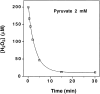
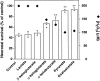
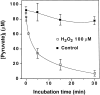
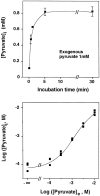
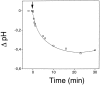
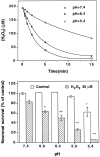
Hydrogen peroxide (H2O2) is suspected to be involved in numerous brain pathologies such as neurodegenerative diseases or in acute injury such as ischemia or trauma. In this study, we examined the ability of pyruvate to improve the survival of cultured striatal neurons exposed for 30 min to H2O2, as estimated 24 hr later by the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide assay. Pyruvate strongly protected neurons against both H2O2 added to the external medium and H2O2 endogenously produced through the redox cycling of the experimental quinone menadione. The neuroprotective effect of pyruvate appeared to result rather from the ability of alpha-ketoacids to undergo nonenzymatic decarboxylation in the presence of H2O2 than from an improvement of energy metabolism. Indeed, several other alpha-ketoacids, including alpha-ketobutyrate, which is not an energy substrate, reproduced the neuroprotective effect of pyruvate. In contrast, lactate, a neuronal energy substrate, did not protect neurons from H2O2. Optimal neuroprotection was achieved with relatively low concentrations of pyruvate (</=1 mM), whereas at high concentration (10 mM) pyruvate was ineffective. This paradox could result from the cytosolic acidification induced by the cotransport of pyruvate and protons into neurons. Indeed, cytosolic acidification both enhanced the H2O2-induced neurotoxicity and decreased the rate of pyruvate decarboxylation by H2O2. Together, these results indicate that pyruvate efficiently protects neurons against both exogenous and endogenous H2O2. Its low toxicity and its capacity to cross the blood-brain barrier open a new therapeutic perspective in brain pathologies in which H2O2 is involved.
Figures
References
-
- Andrae U, Singh J, Ziegler-Skylakakis K. Pyruvate and related α-ketoacids protect mammalian cells in culture against hydrogen peroxide-induced cytotoxicity. Toxicol Lett. 1985;28:93–98. - PubMed
-
- Bakker EP, Van Dam K. The movement of monocarboxylic acids across phospholipid membranes: evidence for an exchange diffusion between pyruvate and other monocarboxylate ions. Biochim Biophys Acta. 1974;339:285–289. - PubMed
-
- Beal MF. Does impairment of energy metabolism result in excitotoxic neuronal death in neurodegenerative illnesses? Ann Neurol. 1992;31:119–130. - PubMed
-
- Berridge MV, Tan AS. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch Biochem Biophys. 1993;303:474–482. - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
