Phosphodiesterase I, a novel adhesion molecule and/or cytokine involved in oligodendrocyte function
- PMID: 9364056
- PMCID: PMC6573621
- DOI: 10.1523/JNEUROSCI.17-23-09095.1997
Phosphodiesterase I, a novel adhesion molecule and/or cytokine involved in oligodendrocyte function
Abstract
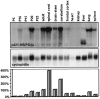
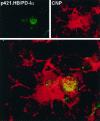
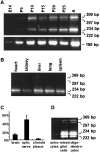
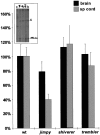
One of the more complex developmental processes occurring postnatally in the CNS is the formation of the myelin sheath by oligodendrocytes. To examine the molecular events that take place during myelination, we isolated oligodendrocyte-derived cDNA clones, one of which (p421.HB) represents a putative alternatively spliced isoform of rat brain-specific phosphodiesterase I (PD-Ialpha) and a species homolog of the human cytokine autotaxin. Analysis of the structural composition of the p421.HB/PD-Ialpha protein suggests a transmembrane-bound ectoenzyme, which, in addition to the phosphodiesterase-active site contains presumed cell recognition and Ca2+-binding domains. Consequently, it may be involved in extracellular signaling events. Expression of p421.HB/PD-Ialpha is enriched in brain and spinal cord, where its mRNA can be detected in oligodendrocytes and in cells of the choroid plexus. Expression in the brain increases during development with an intermediate peak of expression around the time of active myelination and maximal expression in the adult. We have identified four presumably alternatively spliced isoforms, two of which appear to be CNS-specific. Decreased levels of p421.HB/PD-Ialpha mRNA in the dysmyelinating mouse mutant jimpy, but not shiverer, suggest a role for p421.HB/PD-Ialpha during active myelination and/or late stages of oligodendrocyte differentiation. Furthermore, p421.HB/PD-Ialpha mRNA levels were reduced in the CNS at onset of clinical symptoms in experimental autoimmune encephalomyelitis. These data together implicate the importance of p421.HB/PD-Ialpha in oligodendrocyte function, possibly through cell-cell and/or cell-extracellular matrix recognition.
Figures


References
-
- Agresti C, D’Urso D, Levi G. Reversible inhibitory effects of interferon-γ and tumor necrosis factor-α on oligodendroglial lineage cell proliferation and differentiation in vitro. Eur J Neurosci. 1996;8:1106–1116. - PubMed
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Baba H, Fuss B, Watson JB, Zane LT, Macklin WB. Identification of novel mRNAs expressed in oligodendrocytes. Neurochem Res. 1994;19:1091–1099. - PubMed
-
- Babu YS, Bugg CE, Cook WJ. Structure of calmodulin refined 2.2 Å resolution. J Mol Biol. 1988;204:191–204. - PubMed
-
- Barres BA, Hart IK, Coles HSR, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell Death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
