Transplanted oligodendrocyte progenitor cells expressing a dominant-negative FGF receptor transgene fail to migrate in vivo
- PMID: 9364059
- PMCID: PMC6573602
- DOI: 10.1523/JNEUROSCI.17-23-09122.1997
Transplanted oligodendrocyte progenitor cells expressing a dominant-negative FGF receptor transgene fail to migrate in vivo
Abstract
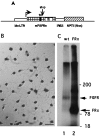
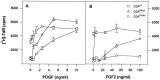
The proliferation, migration, survival, and differentiation of oligodendrocyte progenitor cells, precursors to myelin-forming oligodendrocytes in the CNS, are controlled by a number of polypeptide growth factors in vitro. The requirement and roles for individual factors in vivo, however, are primarily unknown. We have used a cell transplantation approach to examine the role of fibroblast growth factor (FGF) in oligodendrocyte development in vivo. A dominant-negative version of the FGF receptor-1 transgene was introduced into oligodendrocyte progenitors in vitro, generating cells that were nonresponsive to FGF but responsive to other mitogens. When transplanted into the brains of neonatal rats, mutant cells were unable to migrate and remained within the ventricles. These results suggest a role for FGF signaling in establishing a motile phenotype for oligodendrocyte progenitor cell migration in vivo and illustrate the utility of a somatic cell mutagenesis approach for the study of gene function during CNS development in vivo.
Figures





Similar articles
-
Fibroblast growth factor receptor signaling in oligodendrocytes regulates myelin sheath thickness.J Neurosci. 2012 May 9;32(19):6631-41. doi: 10.1523/JNEUROSCI.6005-11.2012. J Neurosci. 2012. PMID: 22573685 Free PMC article.
-
Distinct fibroblast growth factor (FGF)/FGF receptor signaling pairs initiate diverse cellular responses in the oligodendrocyte lineage.J Neurosci. 2005 Aug 10;25(32):7470-9. doi: 10.1523/JNEUROSCI.2120-05.2005. J Neurosci. 2005. PMID: 16093398 Free PMC article.
-
FGF plays a subtle role in oligodendrocyte maintenance in vivo.J Neurosci Res. 1997 Aug 15;49(4):404-15. J Neurosci Res. 1997. PMID: 9285517
-
Fibroblast growth factors and their receptors in oligodendrocyte development: implications for demyelination and remyelination.Dev Neurosci. 2002;24(1):35-46. doi: 10.1159/000064944. Dev Neurosci. 2002. PMID: 12145409 Review.
-
Transplanting oligodendrocyte progenitors into the adult CNS.J Anat. 1997 Jan;190 ( Pt 1)(Pt 1):23-33. doi: 10.1046/j.1469-7580.1997.19010023.x. J Anat. 1997. PMID: 9034879 Free PMC article. Review.
Cited by
-
Extracellular cues influencing oligodendrocyte differentiation and (re)myelination.Exp Neurol. 2016 Sep;283(Pt B):512-30. doi: 10.1016/j.expneurol.2016.03.019. Epub 2016 Mar 23. Exp Neurol. 2016. PMID: 27016069 Free PMC article. Review.
-
Regulation of oligodendrocyte precursor migration during development, in adulthood and in pathology.Cell Mol Life Sci. 2013 Nov;70(22):4355-68. doi: 10.1007/s00018-013-1365-6. Epub 2013 May 21. Cell Mol Life Sci. 2013. PMID: 23689590 Free PMC article. Review.
-
Impaired cerebral cortex development and blood pressure regulation in FGF-2-deficient mice.EMBO J. 1998 Aug 3;17(15):4213-25. doi: 10.1093/emboj/17.15.4213. EMBO J. 1998. PMID: 9687490 Free PMC article.
-
Aberrant growth and differentiation of oligodendrocyte progenitors in neurofibromatosis type 1 mutants.J Neurosci. 2003 Aug 6;23(18):7207-17. doi: 10.1523/JNEUROSCI.23-18-07207.2003. J Neurosci. 2003. PMID: 12904481 Free PMC article.
-
The insulin-like growth factor (IGF) receptor type 1 (IGF1R) as an essential component of the signalling network regulating neurogenesis.Mol Neurobiol. 2009 Dec;40(3):195-215. doi: 10.1007/s12035-009-8081-0. Epub 2009 Aug 29. Mol Neurobiol. 2009. PMID: 19714501 Review.
References
-
- Altman J. Proliferation and migration of undifferentiated precursor cells in the rat during postnatal gliogenesis. Exp Neurol. 1966;16:263–278. - PubMed
-
- Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–270. - PubMed
-
- Ansubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. Greene and Wiley-Interscience; New York: 1988.
-
- Armstrong RC, Harvath L, Dubois-Dalcq M. Type 1 astrocytes and oligodendrocyte-type 2 astrocyte glial progenitors migrate toward distinct molecules. J Neurosci Res. 1990;27:400–407. - PubMed
-
- Baird A. Fibroblast growth factors: activities and significance of non-neurotrophin neurotrophic growth factors. Curr Opin Neurobiol. 1994;4:78–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
