Spatial relationships among three columnar systems in cat area 17
- PMID: 9364073
- PMCID: PMC6573591
- DOI: 10.1523/JNEUROSCI.17-23-09270.1997
Spatial relationships among three columnar systems in cat area 17
Abstract
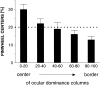
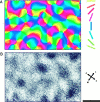
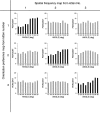
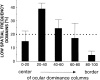
In the primary visual cortex, neurons with similar response properties are arranged in columns. As more and more columnar systems are discovered it becomes increasingly important to establish the rules that govern the geometric relationships between different columns. As a first step to examine this issue we investigated the spatial relationships between the orientation, ocular dominance, and spatial frequency domains in cat area 17. Using optical imaging of intrinsic signals we obtained high resolution maps for each of these stimulus features from the same cortical regions. We found clear relationships between orientation and ocular dominance columns: many iso-orientation lines intersected the borders between ocular dominance borders at right angles, and orientation singularities were concentrated in the center regions of the ocular dominance columns. Similar, albeit weaker geometric relationships were observed between the orientation and spatial frequency domains. The ocular dominance and spatial frequency maps were also found to be spatially related: there was a tendency for the low spatial frequency domains to avoid the border regions of the ocular dominance columns. This specific arrangement of the different columnar systems might ensure that all possible combinations of stimulus features are represented at least once in any given region of the visual cortex, thus avoiding the occurrence of functional blind spots for a particular stimulus attribute in the visual field.
Figures









Similar articles
-
The layout of orientation and ocular dominance domains in area 17 of strabismic cats.Eur J Neurosci. 1998 Aug;10(8):2629-43. doi: 10.1046/j.1460-9568.1998.00274.x. Eur J Neurosci. 1998. PMID: 9767393
-
Ocular dominance peaks at pinwheel center singularities of the orientation map in cat visual cortex.J Neurophysiol. 1997 Jun;77(6):3381-5. doi: 10.1152/jn.1997.77.6.3381. J Neurophysiol. 1997. PMID: 9212282
-
Organization of ocular dominance and orientation columns in the striate cortex of neonatal macaque monkeys.Vis Neurosci. 1995 May-Jun;12(3):589-603. doi: 10.1017/s0952523800008476. Vis Neurosci. 1995. PMID: 7654611
-
Origins of feature selectivities and maps in the mammalian primary visual cortex.Trends Neurosci. 2015 Aug;38(8):475-85. doi: 10.1016/j.tins.2015.06.003. Epub 2015 Jul 22. Trends Neurosci. 2015. PMID: 26209463 Review.
-
Models of orientation and ocular dominance columns in the visual cortex: a critical comparison.Neural Comput. 1995 May;7(3):425-68. doi: 10.1162/neco.1995.7.3.425. Neural Comput. 1995. PMID: 8935959 Review.
Cited by
-
A sub-Riemannian model of the visual cortex with frequency and phase.J Math Neurosci. 2020 Jul 29;10(1):11. doi: 10.1186/s13408-020-00089-6. J Math Neurosci. 2020. PMID: 32728818 Free PMC article.
-
Beyond Rehabilitation of Acuity, Ocular Alignment, and Binocularity in Infantile Strabismus.Front Syst Neurosci. 2018 Jul 18;12:29. doi: 10.3389/fnsys.2018.00029. eCollection 2018. Front Syst Neurosci. 2018. PMID: 30072876 Free PMC article.
-
Patchy distribution of NMDAR1 subunit immunoreactivity in developing visual cortex.J Neurosci. 1998 May 1;18(9):3404-15. doi: 10.1523/JNEUROSCI.18-09-03404.1998. J Neurosci. 1998. PMID: 9547247 Free PMC article.
-
Recent Advances in High-Resolution MR Application and Its Implications for Neurovascular Coupling Research.Front Neuroenergetics. 2010 Sep 27;2:130. doi: 10.3389/fnene.2010.00130. eCollection 2010. Front Neuroenergetics. 2010. PMID: 21048903 Free PMC article.
-
Long-term optical imaging and spectroscopy reveal mechanisms underlying the intrinsic signal and stability of cortical maps in V1 of behaving monkeys.J Neurosci. 2000 Nov 1;20(21):8111-21. doi: 10.1523/JNEUROSCI.20-21-08111.2000. J Neurosci. 2000. PMID: 11050133 Free PMC article.
References
-
- Blasdel GG, Salama G. Voltage-sensitive dyes reveal a modular organization in monkey striate cortex. Nature. 1986;321:579–585. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
