Novel Cdc42-binding proteins Gic1 and Gic2 control cell polarity in yeast
- PMID: 9367980
- PMCID: PMC316694
- DOI: 10.1101/gad.11.22.2972
Novel Cdc42-binding proteins Gic1 and Gic2 control cell polarity in yeast
Abstract
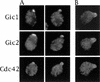
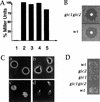
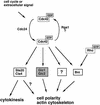
Cdc42p, a Rho-related GTP-binding protein, regulates cytoskeletal polarization and rearrangements in eukaryotic cells, but the effectors mediating this control remain unknown. Through the use of the complete yeast genomic sequence, we have identified two novel Cdc42p targets, Gic1p and Gic2p, which contain consensus Cdc42/Rac interactive-binding (CRIB) domains and bind specifically to Cdc42p-GTP. Gic1p and Gic2p colocalize with Cdc42p as cell polarity is established during the cell cycle and during mating in response to pheromones. Cells deleted for both GIC genes exhibit defects in actin and microtubule polarization similar to those observed in cdc42 mutants. Finally, the interaction of the Gic proteins and Cdc42p is essential, as mutations in the CRIB domain of Gic2p that eliminate Cdc42p binding disrupt Gic2p localization and function. Thus, Gic1p and Gic2p define a novel class of Cdc42p targets that are specifically required for cytoskeletal polarization in vivo.
Figures






References
-
- Aspenström P, Lindberg U, Hall A. Two GTPases, Cdc42 and Rac, bind directly to a protein implicated in the immunodeficiency disorder Wiskott-Aldrich syndrome. Curr Biol. 1996;6:70–75. - PubMed
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. New York, NY: Greene Publishing Associates/Wiley-Interscience; 1991.
-
- Bagrodia S, Derijard B, Davis RJ, Cerione RA. Cdc42 and PAK-mediated signalling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J Biol Chem. 1995;270:27995–27998. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
