The LKLF transcription factor is required for normal tunica media formation and blood vessel stabilization during murine embryogenesis
- PMID: 9367982
- PMCID: PMC316695
- DOI: 10.1101/gad.11.22.2996
The LKLF transcription factor is required for normal tunica media formation and blood vessel stabilization during murine embryogenesis
Abstract
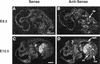
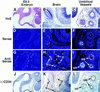
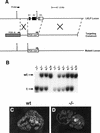
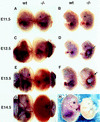
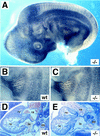
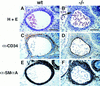
The transcriptional programs that regulate blood vessel formation are largely unknown. In this paper, we examine the role of the zinc finger transcription factor LKLF in murine blood vessel morphogenesis and homeostasis. By in situ hybridization and immunohistochemistry, we show that LKLF is expressed as early as embryonic day 9.5 (E9.5) in vascular endothelial cells throughout the developing mouse embryo. To better understand the function of LKLF, we used homologous recombination in embryonic stem (ES) cells to generate LKLF-deficient (LKLF-/-) mice. Both angiogenesis and vasculogenesis were normal in the LKLF-/- mice. However, LKLF-/- embryos died between E12.5 and E14.5 from severe intra-embryonic and intra-amniotic hemorrhaging. This bleeding disorder was associated with specific defects in blood vessel morphology. Umbilical veins and arteries in the LKLF-/- embryos displayed an abnormally thin tunica media and aneurysmal dilatation before rupturing into the amniotic cavity. Similarly, vascular smooth muscle cells in the aortae from the LKLF-/- animals displayed a cuboidal morphology and failed to organize into a compact tunica media. Consistent with these findings, electron microscopic analyses demonstrated endothelial cell necrosis, significant reductions in the number of vessel-wall pericytes and differentiating smooth muscle cells, and decreased deposition of extracellular matrix in the LKLF-/- vessels. Despite these defects, in situ hybridization demonstrated normal expression of platelet-derived growth factor B, Tie1, Tie2, transforming growth factor beta, and heparin-binding epidermal growth factor in the vasculature of the LKLF-/- embryos. Therefore, LKLF defines a novel transcriptional pathway in which endothelial cells regulate the assembly of the vascular tunica media and concomitant vessel wall stabilization during mammalian embryogenesis.
Figures

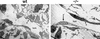

References
-
- Bradley A. Production and analysis of chimeric mice. In: Robertson EJ, editor. Tetratocarcinomas and embryonic stem cells, a practical approach. Oxford, UK: IRL Press Limited; 1987. pp. 113–151.
-
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
