Identification of transcription factories in nuclei of HeLa cells transiently expressing the Us11 gene of herpes simplex virus type 1
- PMID: 9368102
- PMCID: PMC6148282
Identification of transcription factories in nuclei of HeLa cells transiently expressing the Us11 gene of herpes simplex virus type 1
Abstract
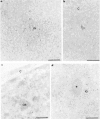
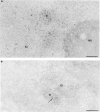
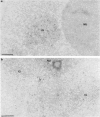
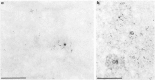
Nuclear distribution and migration of herpes simplex virus type 1 Us11 transcripts were studied in transient expression at the ultrastructural level and compared to that of RNA polymerase II protein. Transcription was monitored by autoradiography following a short pulse with tritiated uridine. Us11 transcripts accumulated mainly over the foci of intermingled RNP fibrils as demonstrated by the presence of silver grains localizing incorporated radioactive uridine superimposed to these structures in which the presence of Us11 RNA and poly(A) tails was previously demonstrated. Silver grains were also scattered over the remaining nucleoplasm but not in the clusters of interchromatin granules, and over the dense fibrillar component of the nucleolus as in control, nontransfected HeLa cells. Pulse-chase experiments revealed the transient presence of migrating RNA in the clusters of interchromatin granules. RNA polymerase II was revealed by immunogold labeling following the use of two monoclonal antibodies: mAb H5, which recognizes the hyperphosphorylated form of the carboxy-terminal domain (CTD) of the molecule, and mAb 7C2, which recognizes both its hyperphosphorylated and unphosphorylated forms. The two mAbs bind to the newly formed Us11 transcription factories and the clusters of interchromatin granules of transfected cells. In control cells, however, clusters of interchromatin granules were labeled with mAb H5 but not with mAB 7C2. Taken together, our data demonstrate the involvement of the clusters of interchromatin granules in the intranuclear migration of Us11 RNA in transient expression. They also suggest the occurrence of changes in the accessibility of the RNA polymerase II CTD upon expression of the Us11 gene after transfection by exposing some epitopes, otherwise masked in nontransfected cells.
Figures


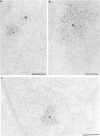
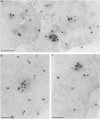
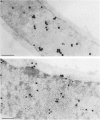
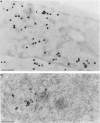
References
-
- Bachellerie J. P.; Puvion E.; Zalta J. P. Ultra-structural organization and biochemical characterization of chromatin-RNA-protein complexes isolated from mammalian cell nuclei. Eur. J. Biochem. 58:327–337; 1975. - PubMed
-
- Bernhard W. A new staining procedure for electron microscopical cytology. J. Ultrastruct. Res. 27:250–265; 1969. - PubMed
-
- Besse S.; Diaz J. J.; Pichard E.; Kindbeiter K.; Madjar J. J.; Puvion-Dutilleul F. In situ hybridization and immuno-electron microscope analyses of herpes simplex virus type 1 US 11 gene during transient expression. Chromosoma 104:434–444; 1996. - PubMed
-
- Bouteille M. The “Ligop” method for routine ultrastructural autoradiography. A combination of a single-coating gold latensification, and phenidon development. J. Microsc. Biol. Cell 27:121–127; 1976.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
