Plasminogen activation in synovial tissues: differences between normal, osteoarthritis, and rheumatoid arthritis joints
- PMID: 9370880
- PMCID: PMC1752434
- DOI: 10.1136/ard.56.9.550
Plasminogen activation in synovial tissues: differences between normal, osteoarthritis, and rheumatoid arthritis joints
Abstract
Objective: To analyse the functional activity of the plasminogen activators urokinase (uPA) and tissue type plasminogen activator (tPA) in human synovial membrane, and to compare the pattern of expression between normal, osteoarthritic, and rheumatoid synovium. The molecular mechanisms underlying differences in PA activities between normal and pathological synovial tissues have been further examined.
Methods: Synovial membranes from seven normal (N) subjects, 14 osteoarthritis (OA), and 10 rheumatoid arthritis (RA) patients were analysed for plasminogen activator activity by conventional zymography and in situ zymography on tissue sections. The tissue distribution of uPA, tPA, uPA receptor (uPAR), and plasminogen activator inhibitor type-1 (PAI-1) was studied by immunohistochemistry. uPA, tPA, uPAR, and PAI-1 mRNA values and mRNA distribution were assessed by northern blot and in situ hybridisations respectively.
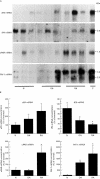
Results: All normal and most OA synovial tissues expressed predominantly tPA catalysed proteolytic activity mainly associated to the synovial vasculature. In some OA, tPA activity was expressed together with variable amounts of uPA mediated activity. By contrast, most RA synovial tissues exhibited considerably increased uPA activity over the proliferative lining areas, while tPA activity was reduced when compared with N and OA synovial tissues. This increase in uPA activity was associated with increased levels of uPA antigen and its corresponding mRNA, which were localised over the synovial proliferative lining areas. In addition, in RA tissues, expression of the specific uPA receptor (uPAR) and of the plasminogen activator inhibitor-type 1
Figures



Similar articles
-
Difference in expression of the plasminogen activation system in synovial tissue of patients with rheumatoid arthritis and osteoarthritis.Br J Rheumatol. 1996 May;35(5):416-23. doi: 10.1093/rheumatology/35.5.416. Br J Rheumatol. 1996. PMID: 8646430
-
Plasminogen activators and inhibitors are transcribed during early macaque implantation.Placenta. 2001 Feb-Mar;22(2-3):186-99. doi: 10.1053/plac.2000.0607. Placenta. 2001. PMID: 11170823
-
Effects of tiaprofenic acid on plasminogen activators and inhibitors in human OA and RA synovium.Br J Rheumatol. 1992;31 Suppl 1:19-26. Br J Rheumatol. 1992. PMID: 1555050
-
uPA/uPAR signaling in rheumatoid arthritis: Shedding light on its mechanism of action.Pharmacol Res. 2018 Aug;134:31-39. doi: 10.1016/j.phrs.2018.05.016. Epub 2018 May 31. Pharmacol Res. 2018. PMID: 29859810 Review.
-
Urokinase in rheumatoid arthritis: causal or coincidental?Ann Rheum Dis. 1997 Dec;56(12):705-6. doi: 10.1136/ard.56.12.705. Ann Rheum Dis. 1997. PMID: 9496148 Free PMC article. Review. No abstract available.
Cited by
-
Enhanced expression of genes involved in coagulation and fibrinolysis in murine arthritis.Arthritis Res. 2000;2(6):504-12. doi: 10.1186/ar132. Epub 2000 Sep 20. Arthritis Res. 2000. PMID: 11056680 Free PMC article.
-
Regulatory polymorphisms in extracellular matrix protease genes and susceptibility to rheumatoid arthritis: a case-control study.Arthritis Res Ther. 2006;8(1):R1. doi: 10.1186/ar1849. Arthritis Res Ther. 2006. PMID: 16356191 Free PMC article.
-
Mactinin: a modulator of the monocyte response to inflammation.Arthritis Res Ther. 2003;5(6):R310-6. doi: 10.1186/ar799. Epub 2003 Aug 5. Arthritis Res Ther. 2003. PMID: 12932295 Free PMC article.
-
Differing roles for urokinase and tissue-type plasminogen activator in collagen-induced arthritis.Am J Pathol. 2002 Mar;160(3):917-26. doi: 10.1016/S0002-9440(10)64914-0. Am J Pathol. 2002. PMID: 11891190 Free PMC article.
-
Development of a Novel Diagnostic Biomarker Set for Rheumatoid Arthritis Using a Proteomics Approach.Biomed Res Int. 2018 Nov 26;2018:7490723. doi: 10.1155/2018/7490723. eCollection 2018. Biomed Res Int. 2018. PMID: 30662913 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

