Proteins on ribosome surface: measurements of protein exposure by hot tritium bombardment technique
- PMID: 9371771
- PMCID: PMC24234
- DOI: 10.1073/pnas.94.24.12892
Proteins on ribosome surface: measurements of protein exposure by hot tritium bombardment technique
Abstract
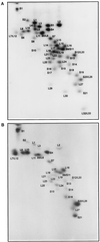
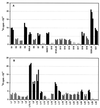
The hot tritium bombardment technique [Goldanskii, V. I., Kashirin, I. A., Shishkov, A. V., Baratova, L. A. & Grebenshchikov, N. I. (1988) J. Mol. Biol. 201,567-574] has been applied to measure the exposure of proteins on the ribosomal surface. The technique is based on replacement of hydrogen by high energy tritium atoms in thin surface layer of macromolecules. Quantitation of tritium radioactivity of each protein has revealed that proteins S1, S4, S5, S7, S18, S20, and S21 of the small subunit, and proteins L7/L12, L9, L10, L11, L16, L17, L24, and L27 of the large subunit are well exposed on the surface of the Escherichia coli 70 S ribosome. Proteins S8, S10, S12, S16, S17, L14, L20, L29, L30, L31, L32, L33, and L34 have virtually no groups exposed on the ribosomal surface. The remaining proteins are found to be exposed to lesser degree than the well exposed ones. No additional ribosomal proteins was exposed upon dissociation of ribosomes into subunits, thus indicating the absence of proteins on intersubunit contacting surfaces.
Figures


References
-
- Frank J, Zhu J, Penczek P, Li Y, Srivastava S, Verschoor A, Radermacher M, Grassucci R, Lata R K, Agraval R K. Nature (London) 1995;376:441–444. - PubMed
-
- Stark H, Orlova E V, Rinke-Appel J, Junke N, Mueller F, Rodnina M, Wintermeyer W, Brimacombe R, van Heel M. Cell. 1997;88:19–28. - PubMed
-
- Klein R, Scheer M. J Phys Chem. 1958;62:1011–1014.
-
- Shishkov A V, Filatov E S, Simonov E F, Unukovich M S, Goldanskii V I, Nesmeyanov A N. Dokl Akad Nauk SSSR. 1976;228:1237–1239. - PubMed
-
- Goldanskii V I, Rumyantsev Yu M, Shishkov A V, Baratova L A, Belyanova L P. Mol Biol (Moscow) 1982;16:528–534. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

