Cloning and expression of a cDNA encoding a bovine brain brefeldin A-sensitive guanine nucleotide-exchange protein for ADP-ribosylation factor
- PMID: 9371777
- PMCID: PMC24240
- DOI: 10.1073/pnas.94.24.12926
Cloning and expression of a cDNA encoding a bovine brain brefeldin A-sensitive guanine nucleotide-exchange protein for ADP-ribosylation factor
Abstract
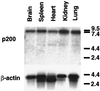
A 200-kDa guanine nucleotide-exchange protein (p200 or GEP) for ADP-ribosylation factors 1 and 3 (ARF1 and ARF3) that was inhibited by brefeldin A (BFA) was purified earlier from cytosol of bovine brain cortex. Amino acid sequences of four tryptic peptides were 47% identical to that of Sec7 from Saccharomyces cerevisiae, which is involved in vesicular trafficking in the Golgi. By using a PCR-based procedure with two degenerate primers representing sequences of these peptides, a product similar in size to Sec7 that contained the peptide sequences was generated. Two oligonucleotides based on this product were used to screen a bovine brain library, which yielded one clone that was a partial cDNA for p200. The remainder of the cDNA was obtained by 5' and 3' rapid amplification of cDNA ends (RACE). The ORF of the cDNA encodes a protein of 1,849 amino acids (approximately 208 kDa) that is 33% identical to yeast Sec7 and 50% identical in the Sec7 domain region. On Northern blot analysis of bovine tissues, a approximately 7.4-kb mRNA was identified that hybridized with a p200 probe; it was abundant in kidney, somewhat less abundant in lung, spleen, and brain, and still less abundant in heart. A six-His-tagged fusion protein synthesized in baculovirus-infected Sf9 cells demonstrated BFA-inhibited GEP activity, confirming that BFA sensitivity is an intrinsic property of this ARF GEP and not conferred by another protein component of the complex from which p200 was originally purified.
Figures

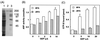
References
-
- Rothman L E. Nature (London) 1994;372:55–63. - PubMed
-
- Brown H A, Gutowski S, Moomaw C R, Slaughter C, Sternweis P C. Cell. 1993;75:1137–1144. - PubMed
-
- Cockcroft S, Thomas G M H, Hensome A, Geny B, Cammingham E, Gout I, Hiles I, Totty N F, Truong O, Hsuan J J. Science. 1994;263:523–526. - PubMed
-
- Moss J, Vaughan M. J Biol Chem. 1995;267:7064–7068. - PubMed
-
- Welsh C F, Moss J, Vaughan M. Mol Cell Biochem. 1994;138:157–166. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

