Smad8 mediates the signaling of the ALK-2 [corrected] receptor serine kinase
- PMID: 9371779
- PMCID: PMC24242
- DOI: 10.1073/pnas.94.24.12938
Smad8 mediates the signaling of the ALK-2 [corrected] receptor serine kinase
Erratum in
- Proc Natl Acad Sci U S A 1998 Feb 17;95(4):1968
Abstract
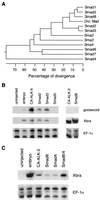
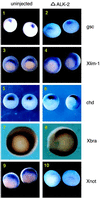
Smad proteins are critical intracellular mediators of signaling by growth and differentiation factors of the transforming growth factor beta superfamily. We have isolated a member of the Smad family, Smad8, from a rat brain cDNA library and biochemically and functionally characterized its ability to transduce signals from serine kinase receptors. In Xenopus embryo, Smad8 is able to transcriptionally activate a subset of mesoderm target genes similar to those induced by the receptor serine kinase, activin receptor-like kinase (ALK)-2. Smad8 can be specifically phosphorylated by a constitutively active ALK-2 but not the related receptor serine kinase, ALK-4. In response to signaling from ALK-2, Smad8 associates with a common regulatory molecule, Smad4, and this association leads to a synergistic effect on gene transcription. Furthermore, Smad8 is able to rescue the expression of mesoderm genes blocked by truncated ALK-2 in the embryo. These results indicate that Smad8 can function as a downstream signaling mediator of ALK-2.
Figures

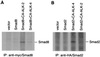

References
-
- Mathews L S, Vale W W. Cell. 1991;65:973–982. - PubMed
-
- Lin H Y, Wang X F, Ng-Eaton E, Weinberg R A, Lodish H F. Cell. 1992;68:775–785. - PubMed
-
- Attisano L, Wrana J L, Lopez C F, Massague J. Biochim Biophys Acta. 1994;1222:71–80. - PubMed
-
- Wrana J L, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M, Wang X F, Massague J. Genes Dev. 1996;10:1880–1889. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

