Erythrocyte membrane vesiculation: model for the molecular mechanism of protein sorting
- PMID: 9371784
- PMCID: PMC24247
- DOI: 10.1073/pnas.94.24.12969
Erythrocyte membrane vesiculation: model for the molecular mechanism of protein sorting
Abstract
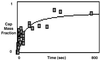
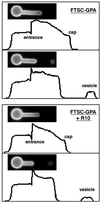
Budding and vesiculation of erythrocyte membranes occurs by a process involving an uncoupling of the membrane skeleton from the lipid bilayer. Vesicle formation provides an important means whereby protein sorting and trafficking can occur. To understand the mechanism of sorting at the molecular level, we have developed a micropipette technique to quantify the redistribution of fluorescently labeled erythrocyte membrane components during mechanically induced membrane deformation and vesiculation. Our previous studies indicated that the spectrin-based membrane skeleton deforms elastically, producing a constant density gradient during deformation. Our current studies showed that during vesiculation the skeleton did not fragment but rather retracted to the cell body, resulting in a vesicle completely depleted of skeleton. These local changes in skeletal density regulated the sorting of nonskeletal membrane components. Highly mobile membrane components, phosphatidylethanolamine- and glycosylphosphatidylinositol-linked CD59 with no specific skeletal association were enriched in the vesicle. In contrast, two components with known specific skeletal association, band 3 and glycophorin A, were differentially depleted in vesicles. Increasing the skeletal association of glycophorin A by liganding its extrafacial domain reduced the fraction partitioning to the vesicle. We conclude that this technique of bilayer/skeleton uncoupling provides a means with which to study protein sorting driven by changes in local skeletal density. Moreover, it is the interaction of particular membrane components with the spectrin-based skeleton that determines molecular partitioning during protein sorting.
Figures



References
-
- Ciechanover A, Schwartz A L, Dautry-Varsat A, Lodish H F. J Biol Chem. 1983;258:9681–9689. - PubMed
-
- Horton M A. Exp Cell Res. 1983;144:361–366. - PubMed
-
- Okumura N, Tsuji K, Nakahata T. Blood. 1992;80:642–650. - PubMed
-
- Vuillett-Gaugler M, Breton-Gorius J, Vainchenker W, Guichard J, Leroy C, Tchernia G, Coulombel L. Blood. 1990;75:865–873. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

