The maturity-onset diabetes of the young (MODY1) transcription factor HNF4alpha regulates expression of genes required for glucose transport and metabolism
- PMID: 9371825
- PMCID: PMC24288
- DOI: 10.1073/pnas.94.24.13209
The maturity-onset diabetes of the young (MODY1) transcription factor HNF4alpha regulates expression of genes required for glucose transport and metabolism
Abstract
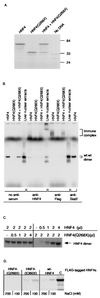
Hepatocyte nuclear factor 4alpha (HNF4alpha) plays a critical role in regulating the expression of many genes essential for normal functioning of liver, gut, kidney, and pancreatic islets. A nonsense mutation (Q268X) in exon 7 of the HNF4alpha gene is responsible for an autosomal dominant, early-onset form of non-insulin-dependent diabetes mellitus (maturity-onset diabetes of the young; gene named MODY1). Although this mutation is predicted to delete 187 C-terminal amino acids of the HNF4alpha protein the molecular mechanism by which it causes diabetes is unknown. To address this, we first studied the functional properties of the MODY1 mutant protein. We show that it has lost its transcriptional transactivation activity, fails to dimerize and bind DNA, implying that the MODY1 phenotype is because of a loss of HNF4alpha function. The effect of loss of function on HNF4alpha target gene expression was investigated further in embryonic stem cells, which are amenable to genetic manipulation and can be induced to form visceral endoderm. Because the visceral endoderm shares many properties with the liver and pancreatic beta-cells, including expression of genes for glucose transport and metabolism, it offers an ideal system to investigate HNF4-dependent gene regulation in glucose homeostasis. By exploiting this system we have identified several genes encoding components of the glucose-dependent insulin secretion pathway whose expression is dependent upon HNF4alpha. These include glucose transporter 2, and the glycolytic enzymes aldolase B and glyceraldehyde-3-phosphate dehydrogenase, and liver pyruvate kinase. In addition we have found that expression of the fatty acid binding proteins and cellular retinol binding protein also are down-regulated in the absence of HNF4alpha. These data provide direct evidence that HNF4alpha is critical for regulating glucose transport and glycolysis and in doing so is crucial for maintaining glucose homeostasis.
Figures



References
-
- Sladek F M, Zhong W, Lai E, Darnell J E., Jr Genes Dev. 1990;4:2353–2365. - PubMed
-
- Sladek F M. In: Liver Gene Transcription. Tronche F, Yaniv M, editors. Austin, TX: Landes; 1994. pp. 207–233.
-
- Chen W S, Manova K, Weinstein D C, Duncan S A, Plump A S, Prezioso V R, Bachvarova R F, Darnell J E., Jr Genes Dev. 1994;8:2466–2477. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

