ATPases and phosphate exchange activities in magnesium chelatase subunits of Rhodobacter sphaeroides
- PMID: 9371849
- PMCID: PMC24312
- DOI: 10.1073/pnas.94.24.13351
ATPases and phosphate exchange activities in magnesium chelatase subunits of Rhodobacter sphaeroides
Abstract
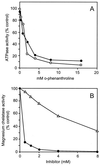
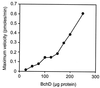
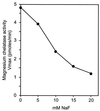
Three separate proteins, BchD, BchH, and BchI, together with ATP, insert magnesium into protoporphyrin IX. An analysis of ATP utilization by the subunits revealed the following: BchH catalyzed ATP hydrolysis at the rate of 0.9 nmol per min per mg of protein. BchI and BchD, tested individually, had no ATPase activity but, when combined, hydrolyzed ATP at the rate of 117.9 nmol/min per mg of protein. Magnesium ions were required for the ATPase activities of both BchH and BchI+D, and these activities were inhibited 50% by 2 mM o-phenanthroline. BchI additionally catalyzed a phosphate exchange reaction from ATP and ADP. We conclude that ATP hydrolysis by BchI+D is required for an activation step in the magnesium chelatase reaction, whereas ATPase activity of BchH and the phosphate exchange activity of BchI participate in subsequent reactions leading to the insertion of Mg2+ into protoporphyrin IX.
Figures

References
-
- Wakao N, Yokoi N, Isoyama N, Hiraishi A, Shimada K, Kobayashi M, Kise H, Iwaki M, Itoh S, Takaichi S, Sakurai Y. Plant Cell Physiol. 1996;37:889–893.
-
- Thauer R K, Bonacker L G. In: The Biosynthesis of the Tetrapyrrole Pigments, Ciba Foundation Symposium 180. Chadwick D J, Ackrill K, editors. Chichester, England: Wiley; 1994. pp. 210–227. - PubMed
-
- Labbe-Bois R, Camadro J-M. In: Metal Ions in Fungi. Winkelman G, Winge D R, editors. New York: Dekker; 1994. pp. 413–453.
-
- Willows R D, Gibson L C, Kannangara C G, Hunter C N, von Wettstein D. Eur J Biochem. 1996;235:438–443. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

