Cisapride reduces neonatal postoperative ileus: randomised placebo controlled trial
- PMID: 9377133
- PMCID: PMC1720698
- DOI: 10.1136/fn.77.2.f119
Cisapride reduces neonatal postoperative ileus: randomised placebo controlled trial
Abstract
Aim: To assess the efficacy of cisapride in reducing ileus persisting to the tenth postoperative day after neonatal abdominal surgery.
Methods: A prospective, randomised, double blind trial comparing rectal cisapride (1.4-2.3 mg/kg/day) with placebo over seven days was undertaken in 33 neonates.
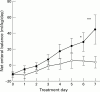
Results: Seven of 12 (58%) patients receiving placebo and eight of 11 (73%) receiving cisapride achieved a first sustained feed during treatment. Of those receiving cisapride, the first sustained feed occurred at 2.3 days (SEM 0.6) compared with 4.7 days (SEM 0.8) with placebo. By the seventh day the mean daily net enteral balance was 69 (SEM 18) ml/kg in the cisapride subgroup and 17 (SEM 8) ml/kg for those receiving placebo. Stool was passed on 6.3 (SEM 0.4) treatment days in the cisapride subgroup compared with 4.1 (SEM 1.0) treatment days in the placebo subgroup.
Conclusion: Cisapride is effective in neonates with a prolonged ileus after abdominal surgery.
Figures



Comment in
-
The use of cisapride in neonates.Arch Dis Child Fetal Neonatal Ed. 2000 Jul;83(1):F75. doi: 10.1136/fn.83.1.f74c. Arch Dis Child Fetal Neonatal Ed. 2000. PMID: 10917723 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
