Permeability of wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride channels to polyatomic anions
- PMID: 9379168
- PMCID: PMC2229373
- DOI: 10.1085/jgp.110.4.355
Permeability of wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride channels to polyatomic anions
Abstract
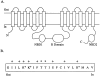
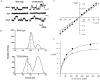
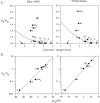
Permeability of the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel to polyatomic anions of known dimensions was studied in stably transfected Chinese hamster ovary cells by using the patch clamp technique. Biionic reversal potentials measured with external polyatomic anions gave the permeability ratio (P/P) sequence NO > Cl > HCO > formate > acetate. The same selectivity sequence but somewhat higher permeability ratios were obtained when anions were tested from the cytoplasmic side. Pyruvate, propanoate, methane sulfonate, ethane sulfonate, and gluconate were not measurably permeant (P/P < 0.06) from either side of the membrane. The relationship between permeability ratios from the outside and ionic diameters suggests a minimum functional pore diameter of approximately 5.3 A. Permeability ratios also followed a lyotropic sequence, suggesting that permeability is dependent on ionic hydration energies. Site-directed mutagenesis of two adjacent threonines in TM6 to smaller, less polar alanines led to a significant (24%) increase in single channel conductance and elevated permeability to several large anions, suggesting that these residues do not strongly bind permeating anions, but may contribute to the narrowest part of the pore.
Figures




References
-
- Anderson MP, Gregory RJ, Thompson S, Souza DW, Paul S, Mulligan RC, Smith AE, Welsh MJ. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science (Wash DC) 1991;253:202–205. - PubMed
-
- Bajnath RB, Groot JA, De Jonge HR, Kansen M, Bijman J. Synergistic activation of non-rectifying small-conductance chloride channels by forskolin and phorbol esters in cell- attached patches of the human colon carcinoma cell line HT-29cl.19A. Pflügers Arch. 1993;425:100–108. - PubMed
-
- Bell CL, Quinton PM. T84 cells: anion selectivity demonstrates expression of Cl−conductance affected in cystic fibrosis. Am J Physiol. 1992;262:C555–C562. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

