Structural regions of the cardiac Ca channel alpha subunit involved in Ca-dependent inactivation
- PMID: 9379170
- PMCID: PMC2229381
- DOI: 10.1085/jgp.110.4.379
Structural regions of the cardiac Ca channel alpha subunit involved in Ca-dependent inactivation
Abstract
We investigated the molecular basis for Ca-dependent inactivation of the cardiac L-type Ca channel. Transfection of HEK293 cells with the wild-type alpha or its 3' deletion mutant (alpha) produced channels that exhibited prominent Ca-dependent inactivation. To identify structural regions of alpha involved in this process, we analyzed chimeric alpha subunits in which one of the major intracellular domains of alpha was replaced by the corresponding region from the skeletal muscle alpha subunit (which lacks Ca-dependent inactivation). Replacing the NH terminus or the III-IV loop of alpha with its counterpart from alpha had no appreciable effect on Ca channel inactivation. In contrast, replacing the I-II loop of alpha with the corresponding region from alpha dramatically slowed the inactivation of Ba currents while preserving Ca-dependent inactivation. A similar but less pronounced result was obtained with a II-III loop chimera. These results suggest that the I-II and II-III loops of alpha may participate in the mechanism of Ca-dependent inactivation. Replacing the final 80% of the COOH terminus of alpha with the corresponding region from alpha completely eliminated Ca-dependent inactivation without affecting inactivation of Ba currents. Significantly, Ca-dependent inactivation was restored to this chimera by deleting a nonconserved, 211-amino acid segment from the end of the COOH terminus. These results suggest that the distal COOH terminus of alpha can block Ca-dependent inactivation, possibly by interacting with other proteins or other regions of the Ca channel. Our findings suggest that structural determinants of Ca-dependent inactivation are distributed among several major cytoplasmic domains of alpha.
Figures
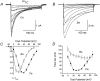
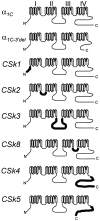
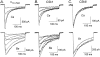
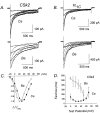

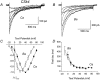


References
-
- Adams B, Tanabe T. Calcium-dependent inactivation of heterologously expressed cardiac L-type calcium channel. Biophys J. 1996;70:238a. . (Abstr.) - PubMed
-
- Beam KG, Adams BA, Niidome T, Numa S, Tanabe T. Function of a truncated dihydropyridine receptor as both voltage sensor and calcium channel. Nature (Lond) 1992;360:169–171. - PubMed
-
- Boyett MR, Harrison SM, Janvier NC, McMorn SO, Owen JM, Shui Z. A list of vertebrate cardiac ionic currents: nomenclature, properties, function and cloned equivalents. Cardiovasc Res. 1996;32:455–481. - PubMed
-
- de Leon M, Wang Y, Jones L, Perez-Reyes E, Wei XY, Soong TW, Snutch TP, Yue DT. Essential Ca2+-binding motif for Ca2+-sensitive inactivation of L-type Ca2+channels. Science (Wash DC) 1995;270:1502–1506. - PubMed

