A thymidine triphosphate shape analog lacking Watson-Crick pairing ability is replicated with high sequence selectivity
- PMID: 9380669
- PMCID: PMC23390
- DOI: 10.1073/pnas.94.20.10506
A thymidine triphosphate shape analog lacking Watson-Crick pairing ability is replicated with high sequence selectivity
Abstract
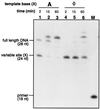
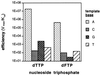
Compound 1 (F), a nonpolar nucleoside analog that is isosteric with thymidine, has been proposed as a probe for the importance of hydrogen bonds in biological systems. Consistent with its lack of strong H-bond donors or acceptors, F is shown here by thermal denaturation studies to pair very poorly and with no significant selectivity among natural bases in DNA oligonucleotides. We report the synthesis of the 5'-triphosphate derivative of 1 and the study of its ability to be inserted into replicating DNA strands by the Klenow fragment (KF, exo- mutant) of Escherichia coli DNA polymerase I. We find that this nucleotide derivative (dFTP) is a surprisingly good substrate for KF; steady-state measurements indicate it is inserted into a template opposite adenine with efficiency (Vmax/Km) only 40-fold lower than dTTP. Moreover, it is inserted opposite A (relative to C, G, or T) with selectivity nearly as high as that observed for dTTP. Elongation of the strand past F in an F-A pair is associated with a brief pause, whereas that beyond A in the inverted A-F pair is not. Combined with data from studies with F in the template strand, the results show that KF can efficiently replicate a base pair (A-F/F-A) that is inherently very unstable, and the replication occurs with very high fidelity despite a lack of inherent base-pairing selectivity. The results suggest that hydrogen bonds may be less important in the fidelity of replication than commonly believed and that nucleotide/template shape complementarity may play a more important role than previously believed.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

