Evidence for two nonidentical drug-interaction sites in the human P-glycoprotein
- PMID: 9380680
- PMCID: PMC23414
- DOI: 10.1073/pnas.94.20.10594
Evidence for two nonidentical drug-interaction sites in the human P-glycoprotein
Abstract
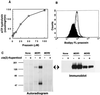
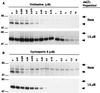
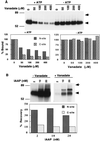
Human P-glycoprotein (Pgp) confers multidrug resistance to cancer cells by ATP-dependent extrusion of a great many structurally dissimilar hydrophobic compounds. The manner in which Pgp recognizes these different substrates is unknown. The protein shows internal homology between its N- and C-terminal halves, each comprised of six putative transmembrane helices and a consensus ATP binding/utilization site. Photoactive derivatives of certain Pgp substrates specifically label two regions, one on each half of the protein. In this study, using [125I]iodoarylazidoprazosin ([125I]IAAP), a photoactive analog of prazosin, we have demonstrated the presence of two nonidentical drug-interaction sites within Pgp. Taking advantage of a highly susceptible trypsin cleavage site in the linker region of Pgp, we characterized the [125I]IAAP binding to the N- and C-terminal halves. cis(Z)-Flupentixol, a modulator of Pgp function, preferentially increased the affinity of [125I]IAAP for the C-terminal half of the protein (C-site) by reducing the Kd from 20 to 6 nM without changing the labeling or affinity (Kd = 42-46 nM) of the N-terminal half (N-site). Also, the concentration of vinblastine (Pgp substrate) and cyclosporin A (Pgp modulator) required for 50% inhibition of [125I]IAAP binding to the C-site was increased 5- to 6-fold by cis(Z)-flupentixol without any effect on the N-site. In addition, [125I]IAAP binding to the N-site was less susceptible than to C-site to inhibition by vanadate which blocks ATP hydrolysis and drug transport. These data demonstrate the presence of at least two nonidentical substrate interaction sites in Pgp.
Figures



References
-
- Gottesman M M, Pastan I. Annu Rev Biochem. 1993;62:385–427. - PubMed
-
- Gottesman M M, Pastan I, Ambudkar S V. Curr Opin Genet Dev. 1996;6:610–617. - PubMed
-
- Gottesman M M, Hrycyna C A, Schoenlein P V, Germann U A, Pastan I. Annu Rev Genet. 1995;29:607–649. - PubMed
-
- Chen C-J, Chin J E, Ueda K, Clark D P, Pastan I, Gottesman M M, Roninson I B. Cell. 1986;47:381–389. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

