B cell receptor-associated protein alpha4 displays rapamycin-sensitive binding directly to the catalytic subunit of protein phosphatase 2A
- PMID: 9380685
- PMCID: PMC23426
- DOI: 10.1073/pnas.94.20.10624
B cell receptor-associated protein alpha4 displays rapamycin-sensitive binding directly to the catalytic subunit of protein phosphatase 2A
Abstract
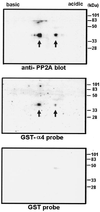
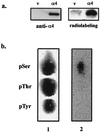
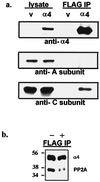
Recently, TAP42 was isolated as a high copy suppressor of sit4-, a yeast phosphatase related to protein phosphatase 2A (PP2A). TAP42 is related to the murine alpha4 protein, which was discovered independently by its association with Ig-alpha in the B cell receptor complex. Herein we show that a glutathione S-transferase (GST)-alpha4 fusion protein bound the catalytic subunit (C) of human PP2A from monomeric or multimeric preparations of PP2A in a "pull-down" assay. In an overlay assay, the GST-alpha4 protein bound to the phosphorylated and unphosphorylated forms of C that were separated in two-dimensional gels and immobilized on filters. The results show direct and exclusive binding of alpha4 to C. This is unusual because all known regulatory B subunits, or tumor virus antigens, bind stably only to the AC dimer of PP2A. The alpha4-C form of PP2A had an increased activity ratio compared with the AC form of PP2A when myelin basic protein phosphorylated by mitogen-activated protein kinase and phosphorylase a were used as substrates. Recombinant alpha4 cleaved from GST was phosphorylated by p56(lck) tyrosine kinase and protein kinase C. A FLAG-tagged alpha4 expressed in COS7 cells was recovered as a protein containing phosphoserine and coimmunoprecipitated with the C but not the A subunit of PP2A. Treatment of cells with rapamycin prevented the association of PP2A with FLAG-alpha4. The results reveal a novel heterodimer alpha4-C form of PP2A that may be involved in rapamycin-sensitive signaling pathways in mammalian cells.
Figures


References
-
- Mumby M C, Walter G. Physiol Rev. 1993;73:673–699. - PubMed
-
- Kamibayashi C, Estes R, Slaughter C, Mumby M C. J Biol Chem. 1991;266:13251–13260. - PubMed
-
- Kamibayashi C, Lickteig R L, Estes R, Walter G, Mumby M C. J Biol Chem. 1992;267:21864–21872. - PubMed
-
- Kamibayashi C, Estes R, Lickteig R L, Yang S I, Craft C, Mumby M C. J Biol Chem. 1994;269:20139–20148. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

