Strain-specific differences in mouse hepatic wound healing are mediated by divergent T helper cytokine responses
- PMID: 9380692
- PMCID: PMC23440
- DOI: 10.1073/pnas.94.20.10663
Strain-specific differences in mouse hepatic wound healing are mediated by divergent T helper cytokine responses
Abstract
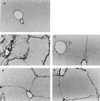
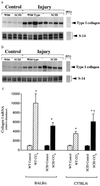
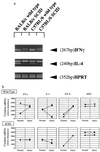
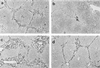
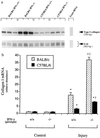
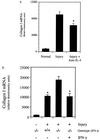
Hepatic fibrosis represents the generalized response of the liver to injury and is characterized by excessive deposition of extracellular matrix. The cellular basis of this process is complex and involves interplay of many factors, of which cytokines are prominent. We have identified divergent fibrosing responses to injury among mouse strains and taken advantage of these differences to examine and contrast T helper (Th)-derived cytokines during fibrogenesis. Liver injury was induced with carbon tetrachloride, fibrosis was quantitated, and Th1/Th2 cytokine mRNAs measured. Liver injury in BALB/c mice resulted in severe fibrosis, whereas C57BL/6 mice developed comparatively minimal fibrosis. Fibrogenesis was significantly modified in T and B cell-deficient BALB/c and C57BL/6 severe combined immunodeficient (SCID) mice compared with wild-type counterparts, suggesting a role of Th subsets. Fibrogenic BALB/c mice exhibited a Th2 response during the wounding response, whereas C57BL/6 mice displayed a Th1 response, suggesting that hepatic fibrosis is influenced by different T helper subsets. Moreover, mice lacking interferon gamma, which default to the Th2 cytokine pathway, exhibited more pronounced fibrotic lesions than did wild-type animals. Finally, shifting of the Th2 response toward a Th1 response by treatment with neutralizing anti-interleukin 4 or with interferon gamma itself ameliorated fibrosis in BALB/c mice. These data support a role for immune modulation of hepatic fibrosis and suggest that Th cytokine subsets can modulate the fibrotic response to injury.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

