Corepressor SMRT binds the BTB/POZ repressing domain of the LAZ3/BCL6 oncoprotein
- PMID: 9380707
- PMCID: PMC23478
- DOI: 10.1073/pnas.94.20.10762
Corepressor SMRT binds the BTB/POZ repressing domain of the LAZ3/BCL6 oncoprotein
Abstract
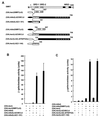
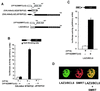
The LAZ3/BCL6 (lymphoma-associated zinc finger 3/B cell lymphomas 6) gene frequently is altered in non-Hodgkin lymphomas. It encodes a sequence-specific DNA binding transcriptional repressor that contains a conserved N-terminal domain, termed BTB/POZ (bric-à-brac tramtrack broad complex/pox viruses and zinc fingers). Using a yeast two-hybrid screen, we show here that the LAZ3/BCL6 BTB/POZ domain interacts with the SMRT (silencing mediator of retinoid and thyroid receptor) protein. SMRT originally was identified as a corepressor of unliganded retinoic acid and thyroid receptors and forms a repressive complex with a mammalian homolog of the yeast transcriptional repressor SIN3 and the HDAC-1 histone deacetylase. Protein binding assays demonstrate that the LAZ3/BCL6 BTB/POZ domain directly interacts with SMRT in vitro. Furthermore, DNA-bound LAZ3/BCL6 recruits SMRT in vivo, and both overexpressed proteins completely colocalize in nuclear dots. Finally, overexpression of SMRT enhances the LAZ3/BCL6-mediated repression. These results define SMRT as a corepressor of LAZ3/BCL6 and suggest that LAZ3/BCL6 and nuclear hormone receptors repress transcription through shared mechanisms involving SMRT recruitment and histone deacetylation.
Figures


References
-
- Kerckaert J P, Deweindt C, Tilly H, Quief S, Lecocq G, Bastard C. Nat Genet. 1993;5:66–70. - PubMed
-
- Ye B H, Lista F, Lo C F, Knowles D M, Offit K, Chaganti R S, Dalla-Favera R. Science. 1993;262:747–750. - PubMed
-
- Miki T, Kawamata N, Hirosawa S, Aoki N. Blood. 1994;83:26–32. - PubMed
-
- Lo Coco F, Ye B H, Lista F, Corradini P, Offit K, Knowles D M, Chaganti R S K, Dalla-Favera R. Blood. 1994;83:1757–1759. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

