Induction of inhibitory factor kappaBalpha mRNA in the central nervous system after peripheral lipopolysaccharide administration: an in situ hybridization histochemistry study in the rat
- PMID: 9380746
- PMCID: PMC23556
- DOI: 10.1073/pnas.94.20.10985
Induction of inhibitory factor kappaBalpha mRNA in the central nervous system after peripheral lipopolysaccharide administration: an in situ hybridization histochemistry study in the rat
Abstract
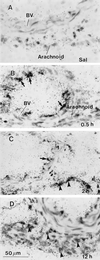
In this study we investigate the mRNA expression of inhibitory factor kappaBalpha (IkappaBalpha) in cells of the rat brain induced by an intraperitoneal (i.p.) injection of lipopolysaccharide (LPS). IkappaB controls the activity of nuclear factor kappaB, which regulates the transcription of many immune signal molecules. The detection of IkappaB induction, therefore, would reveal the extent and the cellular location of brain-derived immune molecules in response to peripheral immune challenges. Low levels of IkappaBalpha mRNA were found in the large blood vessels and in circumventricular organs (CVOs) of saline-injected control animals. After an i.p. LPS injection (2.5 mg/kg), dramatic induction of IkappaBalpha mRNA occurred in four spatio-temporal patterns. Induced signals were first detected at 0.5 hr in the lumen of large blood vessels and in blood vessels of the choroid plexus and CVOs. Second, at 1-2 hr, labeling dramatically increased in the CVOs and choroid plexus and spread to small vascular and glial cells throughout the entire brain; these responses peaked at 2 hr and declined thereafter. Third, cells of the meninges became activated at 2 hr and persisted until 12 hr after the LPS injection. Finally, only at 12 hr, induced signals were present in ventricular ependyma. Thus, IkappaBalpha mRNA is induced in brain after peripheral LPS injection, beginning in cells lining the blood side of the blood-brain barrier and progressing to cells inside brain. The spatiotemporal patterns suggest that cells of the blood-brain barrier synthesize immune signal molecules to activate cells inside the central nervous system in response to peripheral LPS. The cerebrospinal fluid appears to be a conduit for these signal molecules.
Figures




References
-
- Hopkins S J, Rothwell N J. Trends Neurosci. 1995;18:83–88. - PubMed
-
- Muller J M, Ziegler-Heitbrock H W, Baeuerle P A. Immunobiology. 1993;187:233–256. - PubMed
-
- Miyamoto S, Verma I M. Adv Cancer Res. 1995;66:255–292. - PubMed
-
- Cheng Q, Cant C A, Moll T, Hofer-Warbinek R, Wagner E, Birnstiel M L, Bach F H, de Martin R. J Biol Chem. 1994;269:13551–13557. - PubMed
-
- Henkel T, Machleidt T, Alkalay I, Kronke M, Ben-Neriah Y, Baeuerle P A. Nature (London) 1993;365:182–185. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

