Myelin basic protein kinase activity in tomato leaves is induced systemically by wounding and increases in response to systemin and oligosaccharide elicitors
- PMID: 9380763
- PMCID: PMC23618
- DOI: 10.1073/pnas.94.20.11085
Myelin basic protein kinase activity in tomato leaves is induced systemically by wounding and increases in response to systemin and oligosaccharide elicitors
Abstract
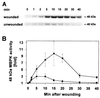
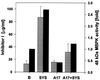
In response to wounding, a 48-kDa myelin basic protein (MBP) kinase is activated within 2 min, both locally and systemically, in leaves of young tomato plants. The activating signal is able to pass through a steam girdle on the stem, indicating that it moves through the xylem and does not require intact phloem tissue. A 48-kDa MBP kinase is also activated by the 18-amino acid polypeptide systemin, a potent wound signal for the synthesis of systemic wound response proteins (swrps). The kinase activation by systemin is strongly inhibited by a systemin analog having a Thr-17 --> Ala-17 substitution, which is a powerful antagonist of systemin activation of swrp genes. A 48-kDa MBP kinase activity also increases in response to polygalacturonic acid and chitosan but not in response to jasmonic acid or phytodienoic acid. In def1, a mutant tomato line having a defective octadecanoid pathway, the 48-kDa MBP kinase is activated by wounding and systemin as in the wild-type plants. This indicates that MBP kinase functions between the perception of primary signals and the DEF1 gene product. In response to wounding, the MBP kinase is phosphorylated on phosphotyrosine residues, indicating a relationship to the mitogen-activated protein kinase family of protein kinases.
Figures




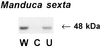

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

