Link between light and fatty acid synthesis: thioredoxin-linked reductive activation of plastidic acetyl-CoA carboxylase
- PMID: 9380765
- PMCID: PMC23628
- DOI: 10.1073/pnas.94.20.11096
Link between light and fatty acid synthesis: thioredoxin-linked reductive activation of plastidic acetyl-CoA carboxylase
Abstract
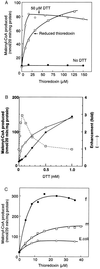
Fatty acid synthesis in chloroplasts is regulated by light. The synthesis of malonyl-CoA, which is catalyzed by acetyl-CoA carboxylase (ACCase) and is the first committed step, is modulated by light/dark. Plants have ACCase in plastids and the cytosol. To determine the possible involvement of a redox cascade in light/dark modulation of ACCase, the effect of DTT, a known reductant of S-S bonds, was examined in vitro for the partially purified ACCase from pea plant. Only the plastidic ACCase was activated by DTT. This enzyme was activated in vitro more efficiently by reduced thioredoxin, which is a transducer of redox potential during illumination, than by DTT alone. Chloroplast thioredoxin-f activated the enzyme more efficiently than thioredoxin-m. The ACCase also was activated by thioredoxin reduced enzymatically with NADPH and NADP-thioredoxin reductase. These findings suggest that the reduction of ACCase is needed for activation of the enzyme, and a redox potential generated by photosynthesis is involved in its activation through thioredoxin as for enzymes of the reductive pentose phosphate cycle. The catalytic activity of ACCase was maximum at pH 8 and 2-5 mM Mg2+, indicating that light-produced changes in stromal pH and Mg2+ concentration modulate ACCase activity. These results suggest that light directly modulates a regulatory site of plastidic prokaryotic form of ACCase via a signal transduction pathway of a redox cascade and indirectly modulates its catalytic activity via stromal pH and Mg2+ concentration. A redox cascade is likely to link between light and fatty acid synthesis, resulting in coordination of fatty acid synthesis with photosynthesis.
Figures


References
-
- Wakil S J, Stoops J K, Joshi V C. Annu Rev Biochem. 1983;52:537–579. - PubMed
-
- Harwood J L. Annu Rev Plant Physiol Plant Mol Biol. 1988;39:101–138.
-
- Woods A, Munday M R, Scott J, Yang X, Carlson M, Carling D. J Biol Chem. 1994;269:19509–19515. - PubMed
-
- Buchanan B B. Annu Rev Plant Physiol. 1980;31:341–374.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

