Functional dissection of COP-I subunits in the biogenesis of multivesicular endosomes
- PMID: 9382865
- PMCID: PMC2140201
- DOI: 10.1083/jcb.139.5.1183
Functional dissection of COP-I subunits in the biogenesis of multivesicular endosomes
Abstract
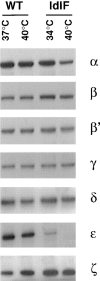
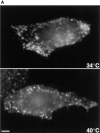
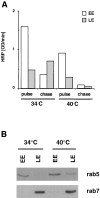
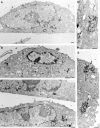
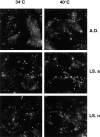
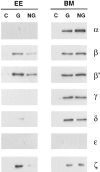
In the present paper, we show that transport from early to late endosomes is inhibited at the restrictive temperature in a mutant CHO cell line (ldlF) with a ts-defect in epsilon coatomer protein (epsilonCOP), although internalization and recycling continue. Early endosomes then appear like clusters of thin tubules devoid of the typical multivesicular regions, which are normally destined to become vesicular intermediates during transport to late endosomes. We also find that the in vitro formation of these vesicles from BHK donor endosomes is inhibited in cytosol prepared from ldlF cells incubated at the restrictive temperature. Although epsilonCOP is rapidly degraded in ldlF cells at the restrictive temperature, cellular amounts of the other COP-I subunits are not affected. Despite the absence of epsilonCOP, we find that a subcomplex of beta, beta', and zetaCOP is still recruited onto BHK endosomes in vitro, and this binding exhibits the characteristic properties of endosomal COPs with respect to stimulation by GTPgammaS and sensitivity to the endosomal pH. Previous studies showed that gamma and deltaCOP are not found on endosomes. However, alphaCOP, which is normally present on endosomes, is no longer recruited when epsilonCOP is missing. In contrast, all COP subunits, except obviously epsilonCOP itself, still bind BHK biosynthetic membranes in a pH-independent manner in vitro. Our observations thus indicate that the biogenesis of multivesicular endosomes is coupled to early endosome organization and depends on COP-I proteins. Our data also show that membrane association and function of endosomal COPs can be dissected: whereas beta, beta', and zetaCOP retain the capacity to bind endosomal membranes, COP function in transport appears to depend on the presence of alpha and/or epsilonCOP.
Figures







References
-
- Bomsel M, Parton R, Kuznetsov SA, Schroer TA, Gruenberg J. Microtubule and motor dependent fusion in vitro between apical and basolateral endocytic vesicles from MDCK cells. Cell. 1990;62:719–731. - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed

