Growth and muscle defects in mice lacking adult myosin heavy chain genes
- PMID: 9382868
- PMCID: PMC2140209
- DOI: 10.1083/jcb.139.5.1219
Growth and muscle defects in mice lacking adult myosin heavy chain genes
Abstract
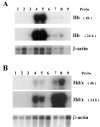
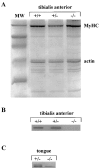
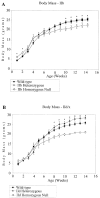
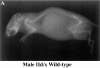
The three adult fast myosin heavy chains (MyHCs) constitute the vast majority of the myosin in adult skeletal musculature, and are >92% identical. We describe mice carrying null mutations in each of two predominant adult fast MyHC genes, IIb and IId/x. Both null strains exhibit growth and muscle defects, but the defects are different between the two strains and do not correlate with the abundance or distribution of each gene product. For example, despite the fact that MyHC-IIb accounts for >70% of the myosin in skeletal muscle and shows the broadest distribution of expression, the phenotypes of IIb null mutants are generally milder than in the MyHC-IId/x null strain. In addition, in a muscle which expresses both IIb and IId/x MyHC in wild-type mice, the histological defects are completely different for null expression of the two genes. Most striking is that while both null strains exhibit physiological defects in isolated muscles, the defects are distinct. Muscle from IIb null mice has significantly reduced ability to generate force while IId null mouse muscle generates normal amounts of force, but has altered kinetic properties. Many of the phenotypes demonstrated by these mice are typical in human muscle disease and should provide insight into their etiology.
Figures





References
-
- Berne, R.M., and M.N. Levy. 1996. Principles of Physiology. Mosby-Year Book, Inc., St. Louis, MO.
-
- Bridges LR, Coulton GR, Howard G, Moss HJ, Mason R. The neuromuscular basis of hereditary kyphoscoliosis in the mouse. Muscle Nerve. 1992;15:172–179. - PubMed
-
- Carter GT, Abresch RT, Fowler WM, Jr, Johnson ER, Kilmer DD, McDonald CM. Profiles of neuromuscular diseases. Spinal muscular atrophy. Am J Phys Med Rehab. 1995;74:150–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

