Neurofilaments and orthograde transport are reduced in ventral root axons of transgenic mice that express human SOD1 with a G93A mutation
- PMID: 9382875
- PMCID: PMC2140205
- DOI: 10.1083/jcb.139.5.1307
Neurofilaments and orthograde transport are reduced in ventral root axons of transgenic mice that express human SOD1 with a G93A mutation
Abstract
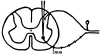
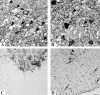
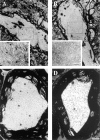
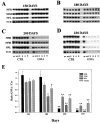
Mice engineered to express a transgene encoding a human Cu/Zn superoxide dismutase (SOD1) with a Gly93 --> Ala (G93A) mutation found in patients who succumb to familial amyotrophic lateral sclerosis (FALS) develop a rapidly progressive and fatal motor neuron disease (MND) similar to amyotrophic lateral sclerosis (ALS). Hallmark ALS lesions such as fragmentation of the Golgi apparatus and neurofilament (NF)-rich inclusions in surviving spinal cord motor neurons as well as the selective degeneration of this population of neurons were also observed in these animals. Since the mechanism whereby mutations in SOD1 lead to MND remains enigmatic, we asked whether NF inclusions in motor neurons compromise axonal transport during the onset and progression of MND in a line of mice that contained approximately 30% fewer copies of the transgene than the original G93A (Gurney et al., 1994). The onset of MND was delayed in these mice compared to the original G93A mice, but they developed the same neuropathologic abnormalities seen in the original G93A mice, albeit at a later time point with fewer vacuoles and more NF inclusions. Quantitative Western blot analyses showed a progressive decrease in the level of NF proteins in the L5 ventral roots of G93A mice and a concomitant reduction in axon caliber with the onset of motor weakness. By approximately 200 d, both fast and slow axonal transports were impaired in the ventral roots of these mice coincidental with the appearance of NF inclusions and vacuoles in the axons and perikarya of vulnerable motor neurons. This is the first demonstration of impaired axonal transport in a mouse model of ALS, and we infer that similar impairments occur in authentic ALS. Based on the temporal correlation of these impairments with the onset of motor weakness and the appearance of NF inclusions and vacuoles in vulnerable motor neurons, the latter lesions may be the proximal cause of motor neuron dysfunction and degeneration in the G93A mice and in FALS patients with SOD1 mutations.
Figures


References
-
- Bannister JV, Bannister WH, Rotilio G. Aspects of the structure, function, and applications of superoxide desmutase. CRC Crit Rev Biochem. 1987;22:111–180. - PubMed
-
- Beyer W, Imlay J, Fridovich I. Superoxide dismutase. Prog Nucleic Acid Res Mol Biol. 1991;40:221–253. - PubMed
-
- Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. - PubMed
-
- Clark EA, Lee VM-Y. Dynamics of mammalian high-molecular-weight neurofilament subunit phosphorylation in cultured rat sympathetic neurons. J Neurosci Res. 1991;30:116–123. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

