Regulation of beta-catenin levels and localization by overexpression of plakoglobin and inhibition of the ubiquitin-proteasome system
- PMID: 9382877
- PMCID: PMC2140206
- DOI: 10.1083/jcb.139.5.1325
Regulation of beta-catenin levels and localization by overexpression of plakoglobin and inhibition of the ubiquitin-proteasome system
Abstract
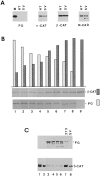
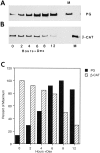
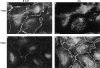
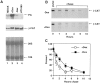
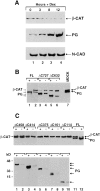
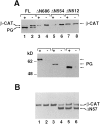
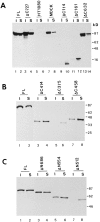
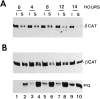
beta-Catenin and plakoglobin (gamma-catenin) are closely related molecules of the armadillo family of proteins. They are localized at the submembrane plaques of cell-cell adherens junctions where they form independent complexes with classical cadherins and alpha-catenin to establish the link with the actin cytoskeleton. Plakoglobin is also found in a complex with desmosomal cadherins and is involved in anchoring intermediate filaments to desmosomal plaques. In addition to their role in junctional assembly, beta-catenin has been shown to play an essential role in signal transduction by the Wnt pathway that results in its translocation into the nucleus. To study the relationship between plakoglobin expression and the level of beta-catenin, and the localization of these proteins in the same cell, we employed two different tumor cell lines that express N-cadherin, and alpha- and beta-catenin, but no plakoglobin or desmosomal components. Individual clones expressing various levels of plakoglobin were established by stable transfection. Plakoglobin overexpression resulted in a dose-dependent decrease in the level of beta-catenin in each clone. Induction of plakoglobin expression increased the turnover of beta-catenin without affecting RNA levels, suggesting posttranslational regulation of beta-catenin. In plakoglobin overexpressing cells, both beta-catenin and plakoglobin were localized at cell-cell junctions. Stable transfection of mutant plakoglobin molecules showed that deletion of the N-cadherin binding domain, but not the alpha-catenin binding domain, abolished beta-catenin downregulation. Inhibition of the ubiquitin-proteasome pathway in plakoglobin overexpressing cells blocked the decrease in beta-catenin levels and resulted in accumulation of both beta-catenin and plakoglobin in the nucleus. These results suggest that (a) plakoglobin substitutes effectively with beta-catenin for association with N-cadherin in adherens junctions, (b) extrajunctional beta-catenin is rapidly degraded by the proteasome-ubiquitin system but, (c) excess beta-catenin and plakoglobin translocate into the nucleus.
Figures



Similar articles
-
Differential interaction of plakoglobin and beta-catenin with the ubiquitin-proteasome system.Oncogene. 2000 Apr 13;19(16):1992-2001. doi: 10.1038/sj.onc.1203519. Oncogene. 2000. PMID: 10803460
-
Differential nuclear translocation and transactivation potential of beta-catenin and plakoglobin.J Cell Biol. 1998 Jun 15;141(6):1433-48. doi: 10.1083/jcb.141.6.1433. J Cell Biol. 1998. PMID: 9628899 Free PMC article.
-
Tyrosine phosphorylation of plakoglobin causes contrary effects on its association with desmosomes and adherens junction components and modulates beta-catenin-mediated transcription.Mol Cell Biol. 2003 Oct;23(20):7391-402. doi: 10.1128/MCB.23.20.7391-7402.2003. Mol Cell Biol. 2003. PMID: 14517306 Free PMC article.
-
Cadherin-catenin complex: protein interactions and their implications for cadherin function.J Cell Biochem. 1996 Jun 15;61(4):514-23. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C514::AID-JCB4%3E3.0.CO;2-R. J Cell Biochem. 1996. PMID: 8806074 Review.
-
Analysis of desmosomal cadherin-adhesive function and stoichiometry of desmosomal cadherin-plakoglobin complexes.J Invest Dermatol. 1996 Sep;107(3):293-300. doi: 10.1111/1523-1747.ep12363000. J Invest Dermatol. 1996. PMID: 8751959 Review.
Cited by
-
Membrane β-catenin and adherens junctions in early gonadal patterning.Dev Dyn. 2012 Nov;241(11):1782-98. doi: 10.1002/dvdy.23870. Epub 2012 Oct 5. Dev Dyn. 2012. PMID: 22972715 Free PMC article.
-
Presenilin 1 negatively regulates beta-catenin/T cell factor/lymphoid enhancer factor-1 signaling independently of beta-amyloid precursor protein and notch processing.J Cell Biol. 2001 Feb 19;152(4):785-94. doi: 10.1083/jcb.152.4.785. J Cell Biol. 2001. PMID: 11266469 Free PMC article.
-
Delta N89 beta-catenin induces precocious development, differentiation, and neoplasia in mammary gland.J Cell Biol. 2001 Apr 30;153(3):555-68. doi: 10.1083/jcb.153.3.555. J Cell Biol. 2001. PMID: 11331306 Free PMC article.
-
β-catenin inhibitors in cancer therapeutics: intricacies and way forward.Bioengineered. 2023 Dec;14(1):2251696. doi: 10.1080/21655979.2023.2251696. Bioengineered. 2023. PMID: 37655825 Free PMC article. Review.
-
Augmented BMP signaling commits cranial neural crest cells to a chondrogenic fate by suppressing autophagic β-catenin degradation.Sci Signal. 2021 Jan 12;14(665):eaaz9368. doi: 10.1126/scisignal.aaz9368. Sci Signal. 2021. PMID: 33436499 Free PMC article.
References
-
- Aberle H, Bierkamp C, Torchard D, Serova O, Wagener T, Natt E, Wirsching J, Heidkämper C, Montagna M, Lynch HT, et al. The human plakoglobin gene localizes on chromosome 17q21 and is subject to loss of heterozygosity in breast and ovarian cancer. Proc Natl Acad Sci USA. 1995;92:6384–6388. - PMC - PubMed
-
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. - PubMed
-
- Ben-Ze'ev A. Cytoskeletal and adhesion proteins as tumor suppressors. Curr Opin Cell Biol. 1997;9:99–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

