In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas
- PMID: 9382881
- PMCID: PMC2199158
- DOI: 10.1084/jem.186.11.1819
In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas
Abstract
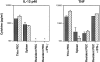
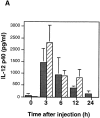
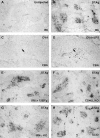
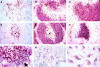
The early induction of interleukin (IL)-12 is a critical event in determining the development of both innate resistance and adaptive immunity to many intracellular pathogens. Previous in vitro studies have suggested that the macrophage (MPhi) is a major source of the initial IL-12 produced upon microbial stimulation and that this response promotes the differentiation of protective T helper cell 1 (Th1) CD4+ lymphocytes from precursors that are primed on antigen-bearing dendritic cells (DC). Here, we demonstrate by immunolocalization experiments and flow cytometric analysis that, contrary to expectation, DC and not MPhi are the initial cells to synthesize IL-12 in the spleens of mice exposed in vivo to an extract of Toxoplasma gondii or to lipopolysaccharide, two well characterized microbial stimulants of the cytokine. Importantly, this production of IL-12 occurs very rapidly and is independent of interferon gamma priming or of signals from T cells, such as CD40 ligand. IL-12 production by splenic DC is accompanied by an increase in number of DCs, as well as a redistribution to the T cell areas and the acquisition of markers characteristic of interdigitating dendritic cells. The capacity of splenic DC but not MPhi to synthesize de novo high levels of IL-12 within hours of exposure to microbial products in vivo, as well as the ability of the same stimuli to induce migration of DC to the T cell areas, argues that DC function simultaneously as both antigen-presenting cells and IL-12 producing accessory cells in the initiation of cell-mediated immunity to intracellular pathogens. This model avoids the need to invoke a three-cell interaction for Th1 differentiation and points to the DC as both a sentinel for innate recognition and the dictator of class selection in the subsequent adaptive response.
Figures


Comment in
-
Interleukin-12, dendritic cells, and the initiation of host-protective mechanisms against Toxoplasma gondii.J Exp Med. 1997 Dec 1;186(11):1799-802. doi: 10.1084/jem.186.11.1799. J Exp Med. 1997. PMID: 9417472 Free PMC article. No abstract available.
References
-
- Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. - PubMed
-
- Hsieh C-S, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science (Wash DC) 1993;260:547–549. - PubMed
-
- Romani L, Mencacci A, Cenci E, Spaccapelo R, Del Sero G, Nicoletti I, Trinchieri G, Bistoni F, Puccetti P. Neutrophil production of IL-12 and IL-10 in candidiasis and efficacy of IL-12 therapy in neutropenic mice. J Immunol. 1997;158:5349–5356. - PubMed
-
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

