Atypical disease after Bordetella pertussis respiratory infection of mice with targeted disruptions of interferon-gamma receptor or immunoglobulin mu chain genes
- PMID: 9382883
- PMCID: PMC2199147
- DOI: 10.1084/jem.186.11.1843
Atypical disease after Bordetella pertussis respiratory infection of mice with targeted disruptions of interferon-gamma receptor or immunoglobulin mu chain genes
Abstract
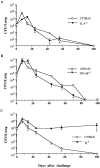
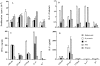
Using a murine respiratory challenge model we have previously demonstrated a role for Th1 cells in natural immunity against Bordetella pertussis, but could not rule out a role for antibody. Here we have demonstrated that B. pertussis respiratory infection of mice with targeted disruptions of the genes for the IFN-gamma receptor resulted in an atypical disseminated disease which was lethal in a proportion of animals, and was characterized by pyogranulomatous inflammation and postnecrotic scarring in the livers, mesenteric lymph nodes and kidneys. Viable virulent bacteria were detected in the blood and livers of diseased animals. An examination of the course of infection in the lung of IFN-gamma receptor-deficient, IL-4-deficient and wild-type mice demonstrated that lack of functional IFN-gamma or IL-4, cytokines that are considered to play major roles in regulating the development of Th1 and Th2 cells, respectively, did not affect the kinetics of bacterial elimination from the lung. In contrast, B cell-deficient mice developed a persistent infection and failed to clear the bacteria after aerosol inoculation. These findings demonstrate an absolute requirement for B cells or their products in the resolution of a primary infection with B. pertussis, but also define a critical role for IFN-gamma in containing bacteria to the mucosal site of infection.
Figures








References
-
- Masure HR. The adenylate cyclase toxin contributes to the survival of Bordetella pertussiswithin human macrophages. Microb Pathog. 1993;14:253–260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

